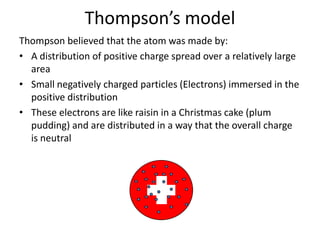
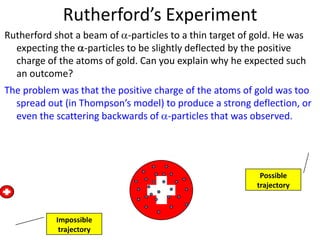
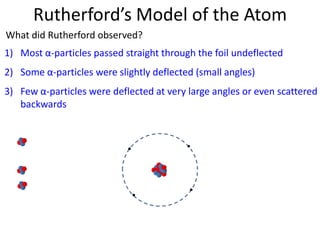
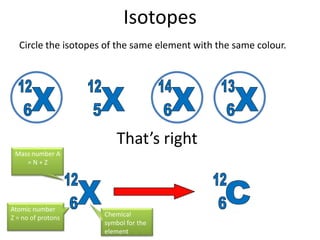
Rutherford's experiment involved shooting alpha particles at a thin gold foil. He expected the particles to be slightly deflected, but instead observed that most passed through undeflected, some were slightly deflected, and a few were scattered backwards. This showed that the atom is mostly empty space, with a small, dense positively charged nucleus at the center surrounded by orbiting electrons. Further experiments revealed protons and neutrons in the nucleus. Isotopes of an element have the same number of protons but different numbers of neutrons, resulting in different atomic masses but the same chemical properties.