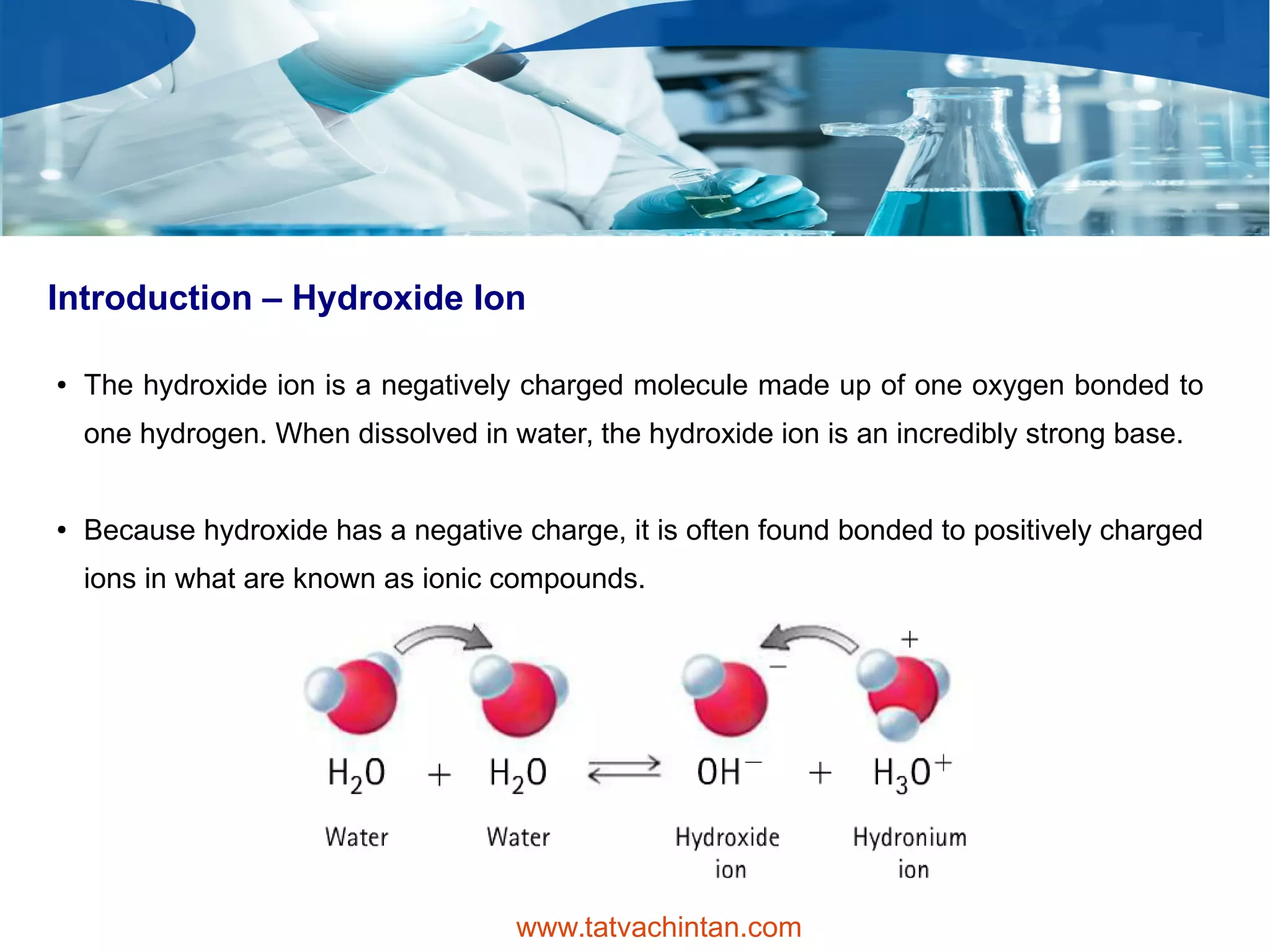
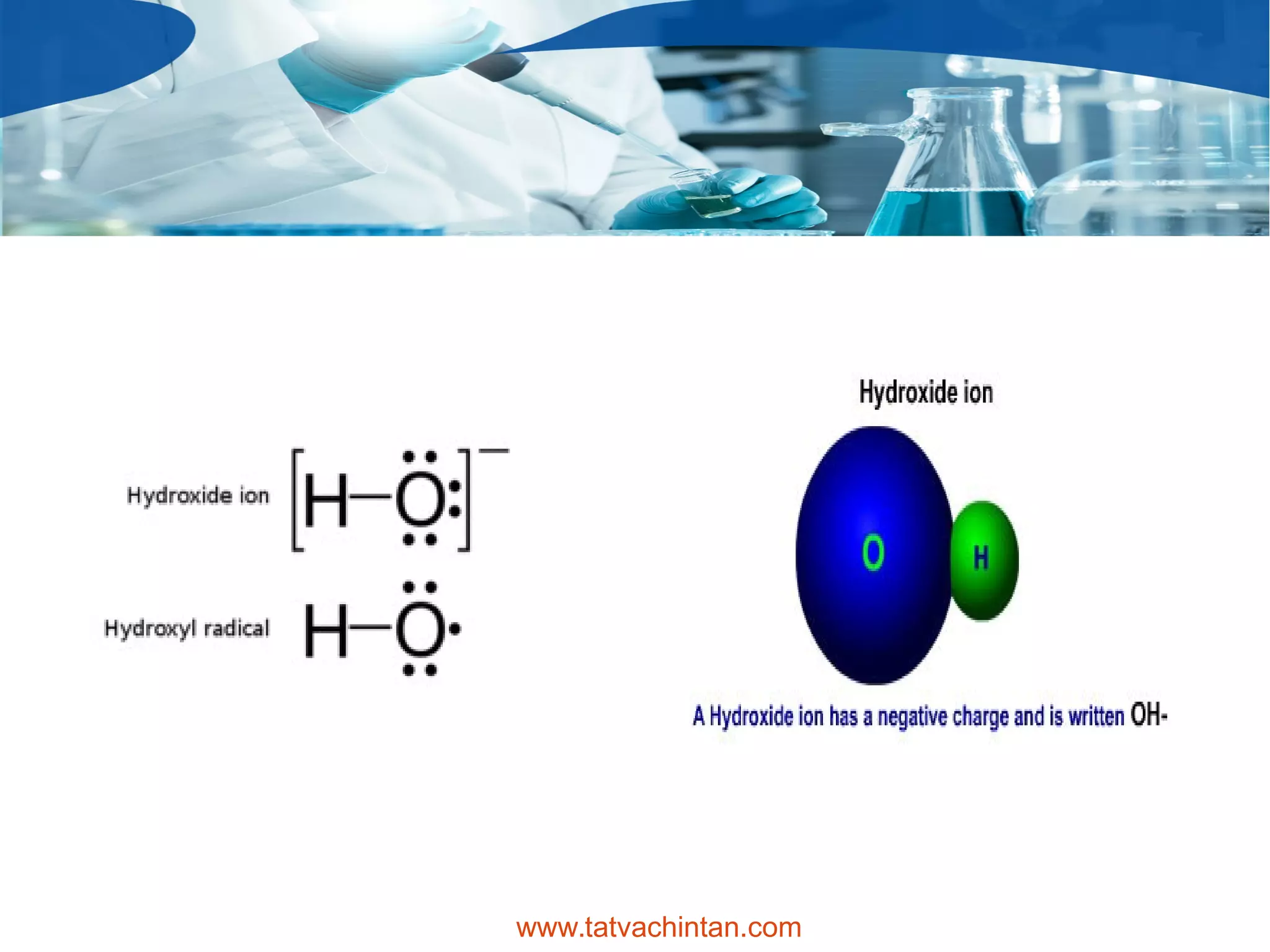
The document describes hydroxides, focusing on the hydroxide ion's properties, its occurrence in ionic compounds, and applications in various industries. It highlights the strong alkalinity of alkali metal hydroxides, usage in cleaning agents, and safety concerns, particularly for workers and children. Additionally, hydroxides are employed in manufacturing plastics, soaps, and in petroleum refining processes.