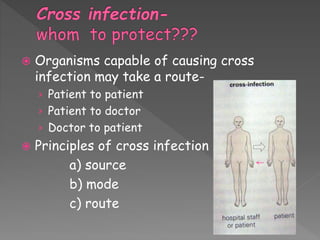
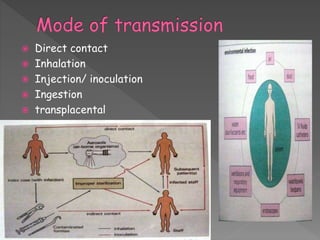
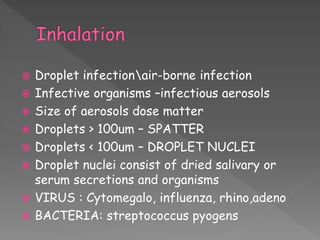
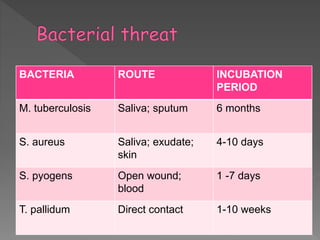
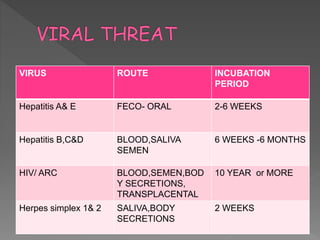
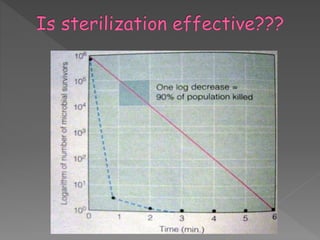
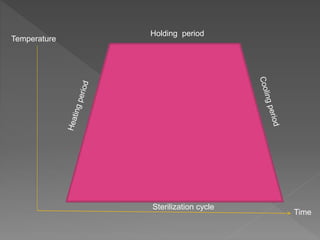
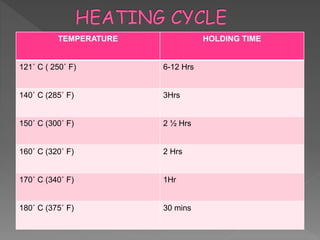
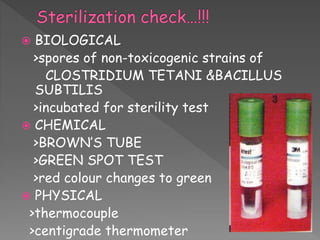
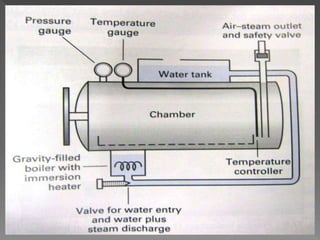
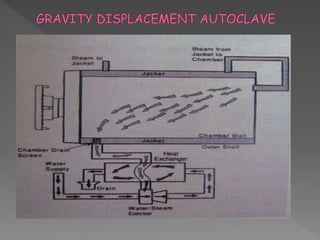
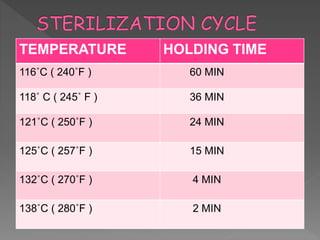
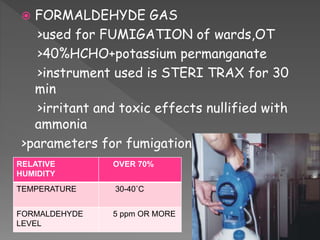
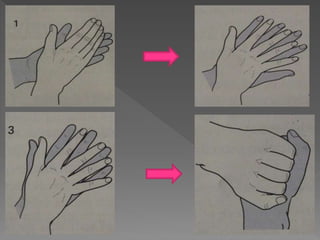
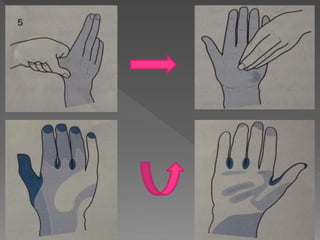
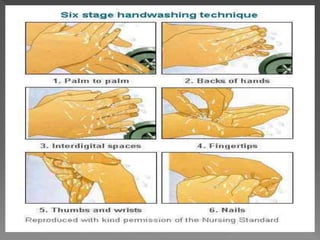
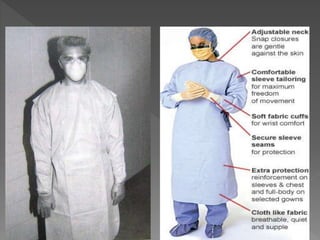
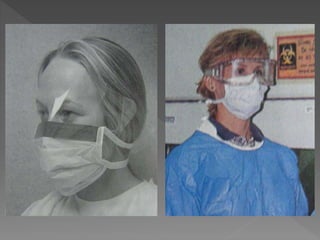
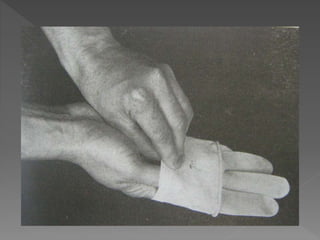
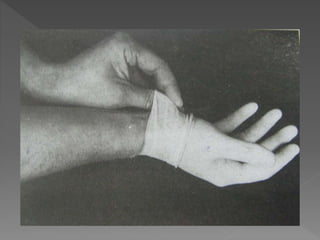
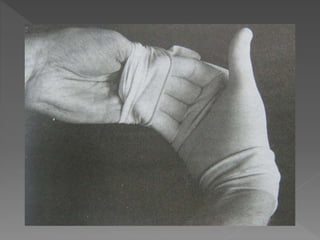
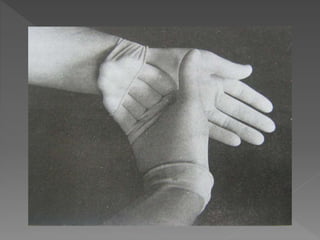
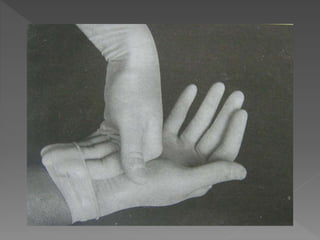
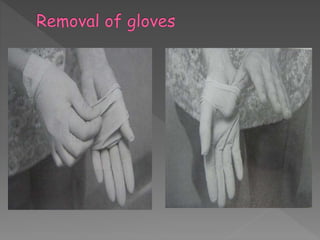
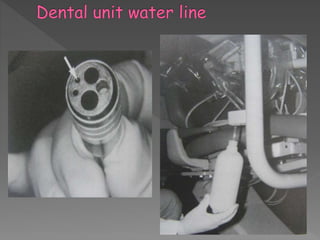
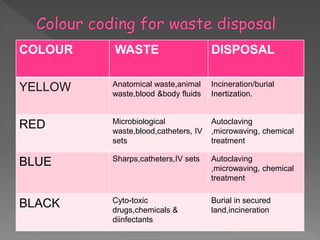
The document discusses infection control and sterilization. It covers various topics like the history of infection control from ancient times through the 19th century pioneers like Semmelweis and Lister. It describes routes of cross-infection and different microorganisms. It also discusses various sterilization methods like heat, chemicals, gases and their mechanisms. It provides details on sterilization of instruments, packaging and monitoring methods. Overall, the document is a comprehensive reference on the concepts, advances and best practices for ensuring infection control and effective sterilization.