Stereospecific and Stereoselective Reactions
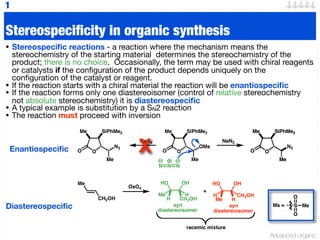
- 1. Advanced organic O Me SiPhMe2 N3 Me O O Me SiPhMe2 N3 Me O N N N O Me SiPhMe2 OMs Me O NaN3 O Me SiPhMe2 N3 Me O NaN3 Stereospecificity in organic synthesis • Stereospecific reactions - a reaction where the mechanism means the stereochemistry of the starting material determines the stereochemistry of the product; there is no choice. Occasionally, the term may be used with chiral reagents or catalysts if the configuration of the product depends uniquely on the configuration of the catalyst or reagent. • If the reaction starts with a chiral material the reaction will be enantiospecific • If the reaction forms only one diastereoisomer (control of relative stereochemistry not absolute stereochemistry) it is diastereospecific • A typical example is substitution by a SN2 reaction • The reaction must proceed with inversion 1 Ms = S O O Me Enantiospecific Diastereospecific Me CH2OH OsO4 HO H Me H CH2OH HO Me H CH2OH H OH OH + syn diastereoisomer syn diastereoisomer racemic mixture X
- 2. Advanced organic Me H OH Me H OH 95.5% (S) 4.5% (R) + Me Me H Ph HO Ph Me Me H Ph HO Ph 91.5% 8.5% + Me Me O H Ph PhMgBr Stereoselectivity in organic synthesis • Stereoselective reactions - a reaction where one stereoisomer of a product is formed preferentially over another. The mechanism does not prevent the formation of two or more stereoisomers but one does predominate. • If a stereogenic centre is introduced into a molecule in such a way that diastereoisomers are produced in unequal amounts the reaction is diastereoselective • If a chemical reaction produces the two enantiomers of a chiral product in unequal amounts it is as an enantioselective reaction 2 Diastereoselective Enantioselective H O Me2Zn (–)-DAIB (2%) Me OH NMe2 Me Me (–)-DAIB 91% ee
- 3. Advanced organic Me O O OMe Me Me Li•H2N(CH2)2NH2 O OMe Me Me Me OH R R S R + >90% Stereospecific reactions • Initially, we will look at the general principles of stereo-specific and -selective reactions • This is intended to familiarize the terminology we have just covered and to instill a number of the basic principles we will be utilising in the rest of the course • In future lectures we will look at ‘asymmetric’ synthesis or various strategies for enantioselective synthesis 3 Enantiospecific reactions Me Me TsO OTs KOH H2S Me Me HS OTs S Me Me Me Me S S R S R R Me Me Me Me • SN2 reaction occurs with complete inversion - retain stereochemical information • Very useful if we already have incorporated stereochemistry • Epoxides are excellent candidates for enantiospecific reactions • Highlights area of potential confusion: (R,S) nomenclature is independent of the ...chemical process occurring (stereochemistry at Me (R) inverted yet still (R) no change in stereochemistry; only name inversion
- 4. Advanced organic Ph H BR2 O OH Ph H OH HOO retention of stereochemistry Ph H BR2 syn addition Ph H BR2 Ph H Ph H O m-CPBA syn Ph H H Ph O m-CPBA anti Ph H H Ph (Z) Ph Ph H H (E) Ph H Ph H O O O H Ar Stereospecific reactions II • Epoxidation with peracids occurs via a concerted process • Results in conservation of alkene geometry Hydroboration • Again occurs via a concerted reaction (bonds made & broken at same time) • Observe syn addition of hydrogen and boron • Further stereospecific transformations possible 4 A number of very useful reactions of alkenes are diastereospecific Electrophilic epoxidation Note: only controlling relative stereocheimstry NOT absolute stereochemistry Note: only controlling relative stereocheimstry NOT absolute stereochemistry
- 5. Advanced organic I Me O O syn O O H Me I2 (Z) I Me O O H I Me O O anti I Me Me Br Br syn rotate central bond Br2 H Me Me H Br Br Me Br Me Br H Me Me H (Z) Me Me Br Br anti Br2 Me H Me H Br Br Me H Me H (E) Stereospecific reactions III Bromination • Bromination of alkenes proceeds with the anti addition of Br2 across the double bond • This is the result of the formation of a bromonium cation followed by SN2 attack • The geometry of the starting material controls the stereochemistry of the product 5 Iodolactonisation • Proceeds in an analogous fashion via an iodonium species • Geometry of alkene controls relative stereochemistry Note: only controlling relative stereocheimstry NOT absolute stereochemistry O O H Me I2 (E) O O H Me I
- 6. Advanced organic view from this face H Ph O 2 3 1 anti-clockwise Si face H Ph O 2 3 1 H Ph O 2 3 1 Stereoselective reactions Nucleophilic addition to C=O • Reaction of a nucleophile with a chiral substrate gives two possible diastereoisomers • Reaction is stereoselective if one diastereoisomer predominates 6 Me R O H Ph LiAlH4 H3O+ Me R H Ph H OH Me R H Ph H OH + R = Me R = t-Bu 75% (50% de) 98% (96% de) 25% 2% : : O Ph H view from this face clockwise Re face Prochiral Nomenclature • Trigonal carbons that are not stereogenic centres but can be made into them are prochiral • Each face can be assigned a label based on the CIP rules • If the molecule is chiral (as above) the faces are said to be diastereotopic • If the molecule is achiral (as below) the faces are enantiotopic % de = diastereisomeric excess = [major] – [minor] [major] + [minor] = %major – %minor
- 7. Advanced organic Ph Me H H O Ph Me H H O Ph H Me H O Ph H Me H O Ph H O Me H Ph H O Me H Ph H Me H O two substituents (C=O & Ph) are eclipsed - unfavoured Felkin-Ahn model • The diastereoselectivity can be explained and predicted via the Felkin-Ahn model • It is all to do with the conformation of the molecule... • Easiest to understand if we look at the Newman projection of the starting material 7 Ph H O Me H EtMgBr Ph Et Me H Ph Et Me H H OH HO H 25% 75% (50% de) + • Rotate around central bond so that substituents are staggered • Two favoured as largest substituent (Ph) furthest from O & H • Continue to rotate around central bond and find 6 possible conformations Me Ph H H O Me H Ph H O H Me Ph H O H Ph Me H O largest substituent (Ph) furthest from O & H largest substituent (Ph) furthest from O & H
- 8. Advanced organic C O R R C O R R Nu C O R R C O R R Nu Felkin-Ahn model II • As a result of the Bürghi-Dunitz (107°) angle there are four possible trajectories for the nucleophile to approach the most stable conformations • Three are disfavoured due to steric hindrance of Ph or Me • Therefore, only one diastereoisomer is favoured 8 • Nucleophiles attack the carbonyl group along the Bürghi-Dunitz angle of ~107° maximum overlap with π* - nucleophile attacks at 90° to C=O C O R R Nu C O R R repulsion from full π orbital - nucleophile attacks from obtuse angle compromise, nucleophile attacks π* orbital at angle of 107° Ph H Me H O Ph Me H H O Nu Nu Nu Nu Bürghi-Dunitz angle: 107° Ph H Me H O Ph Me H H O Nu Nu Nu Nu close to Ph close to Ph close to Me unhindered approach X X X • Favoured approach passed smallest substituent (H) when molecule in most stable ...conformation
- 9. Advanced organic Ph Me H OH Et H Ph Me H OH Et H Ph Et Me H HO H Ph Et Me H HO H Ph Et Me H HO H Ph Et Me H HO H Ph Me H H O Ph H O Me H EtMgBr Felkin-Ahn model III • Apply the Felkin-Ahn model to our example • Most problems seem to occur when swapping between different representations... 9 Ph Et Me H HO H Ph H O Me H EtMgBr 1. So, assuming we have used the Felkin-Ahn model and Newman projections to predict the product, how do we draw the correct ‘zig-zag’ representation? Ph Et Me H HO H Ph H O Me H EtMgBr 2. First, remember which parts of the molecule have not been effected by the reaction and draw them 3. As the original stereogenic centre has not changed, we will compare the relative orientation of the substituents on the new centre to these Ph Me H H HO Et Ph Et Me H HO H Ph H O Me H EtMgBr 4. Remember, we prefer to draw the main carbon chain in the plane of page, therefore, align Ph and Et in Newman projection as well Ph Me H OH Et H 5. Me and OH on same side, therefore, as Me not effected by reaction & is ‘up,’ OH must be ‘up.’ This leaves both H down. Et Ph Me H OH Et H Ph Me H OH Et H rotate
- 10. Advanced organic Felkin-Ahn model IV • To explain or predict the stereoselectivity of nuclophilic addition to a carbonyl group with an adjacent stereogenic centre, use the Felkin-Ahn model • Draw Newman projection with the largest substituent (L) perpendicular to the C=O • Nucleophile (Nu) will attack along the Bürghi-Dunitz trajectory passed the least sterically demanding (smallest, S) substituent • Draw the Newman projection of the product • Redraw the molecule in the normal representation • Whilst the Felkin-Ahn model predicts the orientation of attack, it does not give any information about the degree of selectivity • Many factors can effect this... 10 L R O M S L M S R O Nu L M S OH Nu R L R M S Nu OH L = large group, M = medium group, S = small group
- 11. Advanced organic Diastereoselective addition to carbonyl group • The size of the nucleophile greatly effects the diastereoselectivity of addition • Larger nucleophiles generally give rise to greater diastereoselectivities • Choice of metal effects the selectivity as well, although this may just be a steric effect • The size of substituents on the substrate will also effect the diastereoselectivity • Again, larger groups result in greater selectivity • Should be noted that larger substituents normally result in a slower rate of reaction 11 Ph R Me H HO H Ph H O Me H RMgBr R = Me R = Et R = Ph 40% de 50% de 60% de Ph Me Me H HO H Ph H O Me H Me(metal) Me(metal) = MeMgI Me(metal) = MeTi(OPh)3 33% de 86% de Me R O H Ph Me R H Ph H OH R = Me R = Et R = i-Pr R = t-Bu 50% de 50% de 66% de 96% de LiAlH4
- 12. Advanced organic Effect of electronegative atoms • It is hard to justify the excellent selectivity observed above using simple sterics • The Bn2N group must be perpendicular to C=O but a second factor must explain why the selectivity is so high (& the reaction much faster than previous examples) • There is an electronic effect 12 Me Et NBn2 O H OLi OMe Me Et NBn2 OH OMe O >92% de Bn2N H H O Me Et OLi OMe Bn2N H HO H Me Et CO2Me • Overlap results in a new, lower energy orbital, more susceptible to nucleophilic attack • Thus if electronegative group perpendicular, C=O is more reactive Z Y X O R Nu Z = electro- negative group • When an electronegative group is perpendicular to the C=O it is possible to get an ...overlap of the π* orbital and the σ* orbital Z Y X O R Nu C=O π* C–Z σ* nucleophile interacts with π* orbital C=O π* C–Z σ* new π*+σ* LUMO Z Y X O R Nu new π*+σ* LUMO new low energy orbital formed from C=O & C-Z anti- bonding orbitals favours nucleophilic attack at carbonyl
- 13. Advanced organic Et MeS H Ph O Zn rotate to allow chelation MeS Et H Ph O Ph Et O SMe Zn Ph Et SMe H OH BH4 Ph Et O SMe Li R3BH Ph Et SMe HO H Effect of electronegative atoms II • A good example of this effect is shown • But as always, chemistry not that simple... 13 MeS Et H Ph O H BR3 MeS Et H HO H Ph Felkin-Ahn attack Cram-chelation control Et MeS H OH H Ph H H3B • If heteroatom (Z) is capable of coordination and... ...a metal capable of chelating 2 heteroatoms is present we observe chelation control • Metal chelates carbonyl and heteroatom together • This fixes conformation • Such reactions invariably occur with greater selectivity • Reactions are considerably faster • The chelating metal acts as a Lewis acid and activates the carbonyl group to attack • As shown, chelation can reverse selectivity!
- 14. Advanced organic Chelation control • Chelation controlled additions are easy to predict • Normally do not need to draw Newman projection (yippee!) • Simple example shown below 14 L R Nu OH Z S M R O Z M S L L R O Z S Z = heteroatom capable of coordination; M = metal capable of coordinating to more than one heteroatom Nu Nucleophile attacks from least hindered face O H O Me PhMgI O H Me HO Ph 96% de
- 15. Advanced organic Me Me Ph2PO H OH NaBH4, CeCl3 EtOH, –78°C Me O Me Ph2PO Me Me Ph2PO H OH NaBH4 MeOH, 20°C Chelation control II • Example shows normal Felkin-Ahn selectivity gives one diastereoisomer • Electronegative and bulky phosphorus group in perpendicular position 15 • Chelation control gives opposite diastereoisomer • Chelation can occur through 6-membered ring • Lower temperature typical of activated, chelated carbonyl P Me H O Me O Ph Ph H BH3 Me P H O H H3B Me O Ph Ph Ce