Hammete Equation
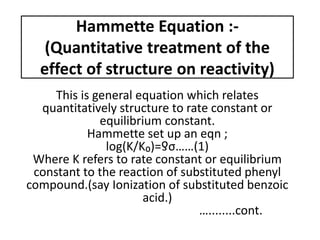
- 1. Hammette Equation :- (Quantitative treatment of the effect of structure on reactivity) This is general equation which relates quantitatively structure to rate constant or equilibrium constant. Hammette set up an eqn ; log(K/K₀)=ƍσ……(1) Where K refers to rate constant or equilibrium constant to the reaction of substituted phenyl compound.(say Ionization of substituted benzoic acid.) …........cont.
- 2. ……..cont. K₀ refers to the same reaction (say ionization of benzoic acid). σ is substituent constant which indicating relative electron withdrawing or releasing group:-affect of substitution on rate of reaction. ƍ is reaction constant for given reaction under given set of reaction. The value of ƍ was set 1 for ionization of substituted and unsubstituted Benzoic acid in water at 25°C and σ values were then calculated. (………….cont…)
- 3. ……cont. Thus, log(K/K₀)=σ……….(2) or, σ = log(KC6H4COOH/K₀C6H5COOH) σ=Effect of substituent on rate of reaction. Substituent have similar effect from reaction to reaction.σ is different for meta and Para position. (………cont……)
- 4. ……cont. Substituent Constant (σ):- It is the number. Since, σ is determined by calculating the logarithm of ionization constant of substituted benzoic acid to the ionization constant of unsubstituted benzoic acid. σ value of H is zero. σ value is measure of total polar effect i.e. it represents the ability of substituent to withdraw or release of electron by the combination of I-effect and Resonance effect of substituent. (…….cont.)
- 5. ……cont. The σ value for substituent of meta position differ from that for substituent at Para position. σ value may be negative or positive number. A negative σ value for a group at particular positions indicates its greater electron donating ability to the reaction centre than hydrogen whereas positive value for substituent indicates its electron withdrawing character. (cont………)
- 6. …………cont. For Example:- K C6H5COOH = 6.3 Х 10^-5 Km MeOC6H4COOH = 8.3 X 10^-5 σm = log (8.3X10^-5/6.3X10^-5) = log8.3 – log 6.3 = 0.9191-0.7993 = +0.12 Hence, m-methoxybenzoic acid has greater electron withdrawing ability than benzoic acid. (cont……….)
- 7. ………………..cont. Reaction Constant (ƍ) :- It indicates relative need of reactions for electron withdraw or release. This depends on the reaction, nature of reaction centre and condition ( pressure, temperature, solvent catalyst etc). ƍ value may be positive or negative. A negative value indicates a large electron demand at the reaction centre. Since, electron donating substituent accelerates the rate of reaction. (cont…………………..)
- 8. ………….cont. A positive value is associated with developing a negative charge in transition state. So, e- withdrawing group accelerates the rate of reaction. Application (Uses of Hammette equation ) :- 1) Since, equation involve four measureable quantities K , K₀ , ƍ & σ. If any three of them are known the fourth can be calculated. (cont..
- 9. ……………cont. 2) To compare the acidity of acids quantitatively :- For e.g. p-nitro phenyl acetic acid (p-NO2 C6H5CH2COOH ) is more acidic than phenyl acetic acid (C6H5CH2COOH) due to electron withdrawing effect of p-nitro group. This can be solved by Hammette equation. - σ for nitro group = +0.78 - ƍ for ionization of C6H5CH2COOH = +0.49 (cont….)
- 10. (….cont.) Log ( ) = σƍ log = 0.78 X 0.49 or, = 10^0.3822 or, = 2.411 (cont…) K p-nitro C6H4CH2COOH K₀C6H5CH2COOH
- 11. (…...cont.) It shows that the p-nitro phenyl acetic acid is expected to be 2.4 times more acidic than phenyl acetic acid. 3) To compare reactivity of two compounds quantitatively by Hammette equation :- For e.g. Reactivity of p-chloro phenyl methyl chloride with that of Benzyl chloride in 50% acetone at 60°C. σ for Cl = +0.23 ƍ = -1.70 (cont…………)
- 12. (…………..cont. ) log = σ X ƍ or, log = 0.23 X - 1.70 or, = - 3.91 Thus, the rate of hydrolysis of p-chloro benzyl chloride is slower than the Benzyl chloride . (cont......)
- 13. (cont…….) 4) ƍ value & the reaction pathway :- From the value of & sign of the reaction constant, we can understand something about the reaction mechanism. For e.g. the reaction constant ƍ for the hydrolysis of Benzyl chloride in 50% acetone & 60°C temperature is -1.70. The negative value of ƍ indicates that the transition state of rate determing step must bear positive charge, the rate determing step of Benzyl chloride into Benzyl cation and SN1 type of reaction involved ionization in rate determing step . So, It follows SN1 path.