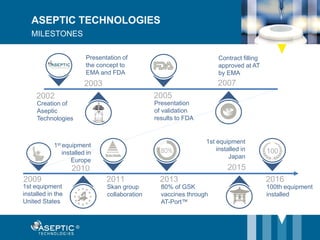
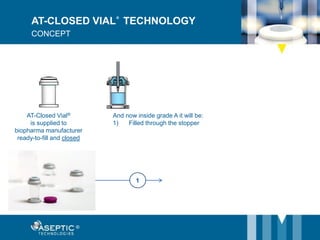
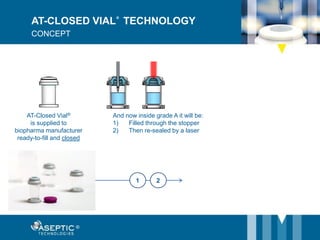
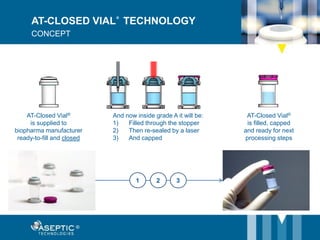
Aseptic Technologies, established in 2002, focuses on innovative aseptic filling solutions for biopharmaceuticals, including technologies for handling cell and gene therapies, vaccines, and antibodies. Their AT-Closed Vial® technology allows biopharma manufacturers to efficiently fill and seal vials under aseptic conditions, and they have achieved significant milestones, including the installation of their 100th filling equipment by 2016. The company continues to enhance its offerings, ensure safety in aseptic operations, and expand its global presence.