Embed presentation
Downloaded 15 times

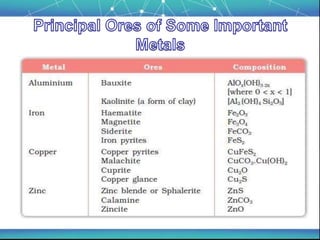




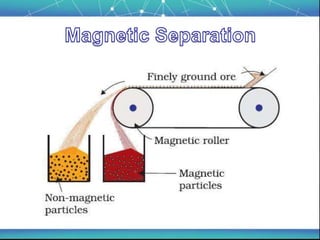

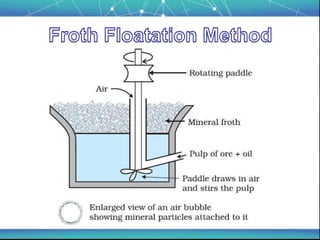
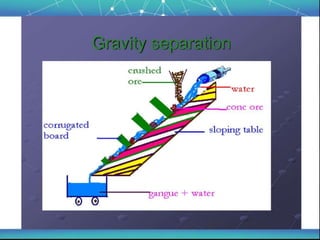
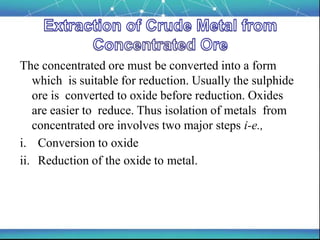
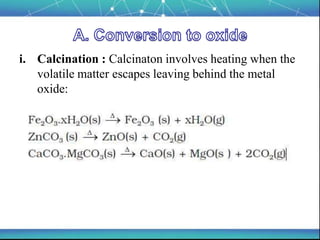
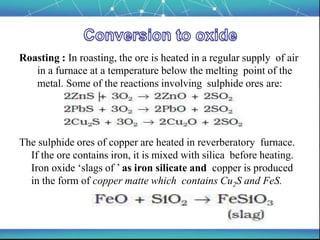
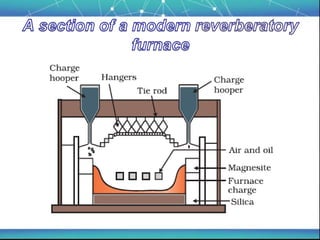

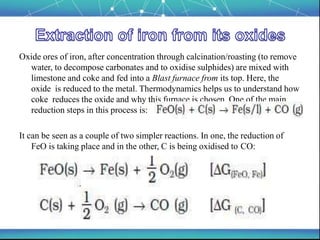
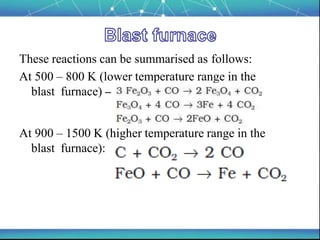

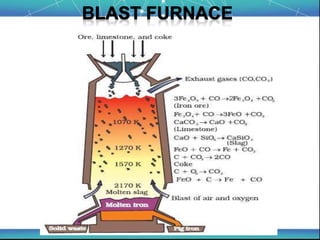

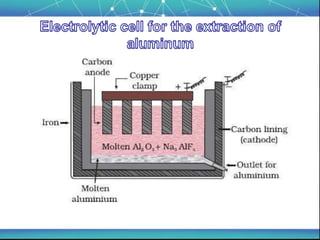





The document discusses the extraction of metals, focusing on aluminum and iron as the most abundant metals in the Earth's crust. It outlines the processes of ore concentration, conversion to oxides, and reduction to metals, detailing various methods such as magnetic separation, froth flotation, and thermodynamic principles applied in blast furnaces. Additionally, it covers techniques for purifying metals post-extraction, including distillation and electrolysis.


























