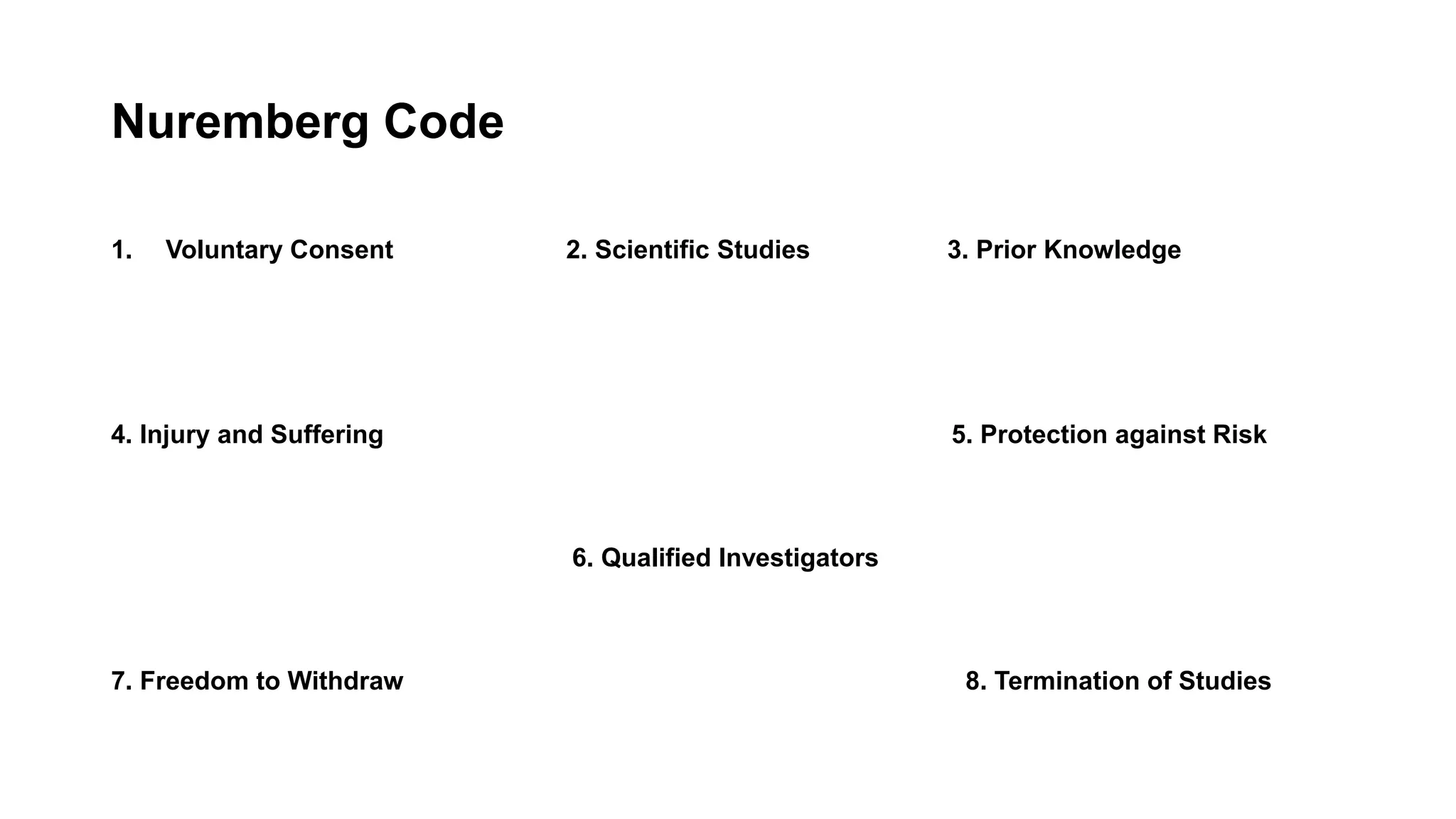
The document summarizes the history and key principles of the Nuremberg Code, which was established after World War II in response to unethical medical experiments conducted by Nazi doctors on concentration camp prisoners without consent. The Nuremberg Code set forth 10 ethical principles for human experimentation, including requirements for voluntary and informed consent, that experiments yield socially useful results not obtainable by other means, and that risks to subjects be avoided wherever possible. It had a significant influence on later guidelines for ethical clinical research involving human subjects.