Embed presentation
Download to read offline







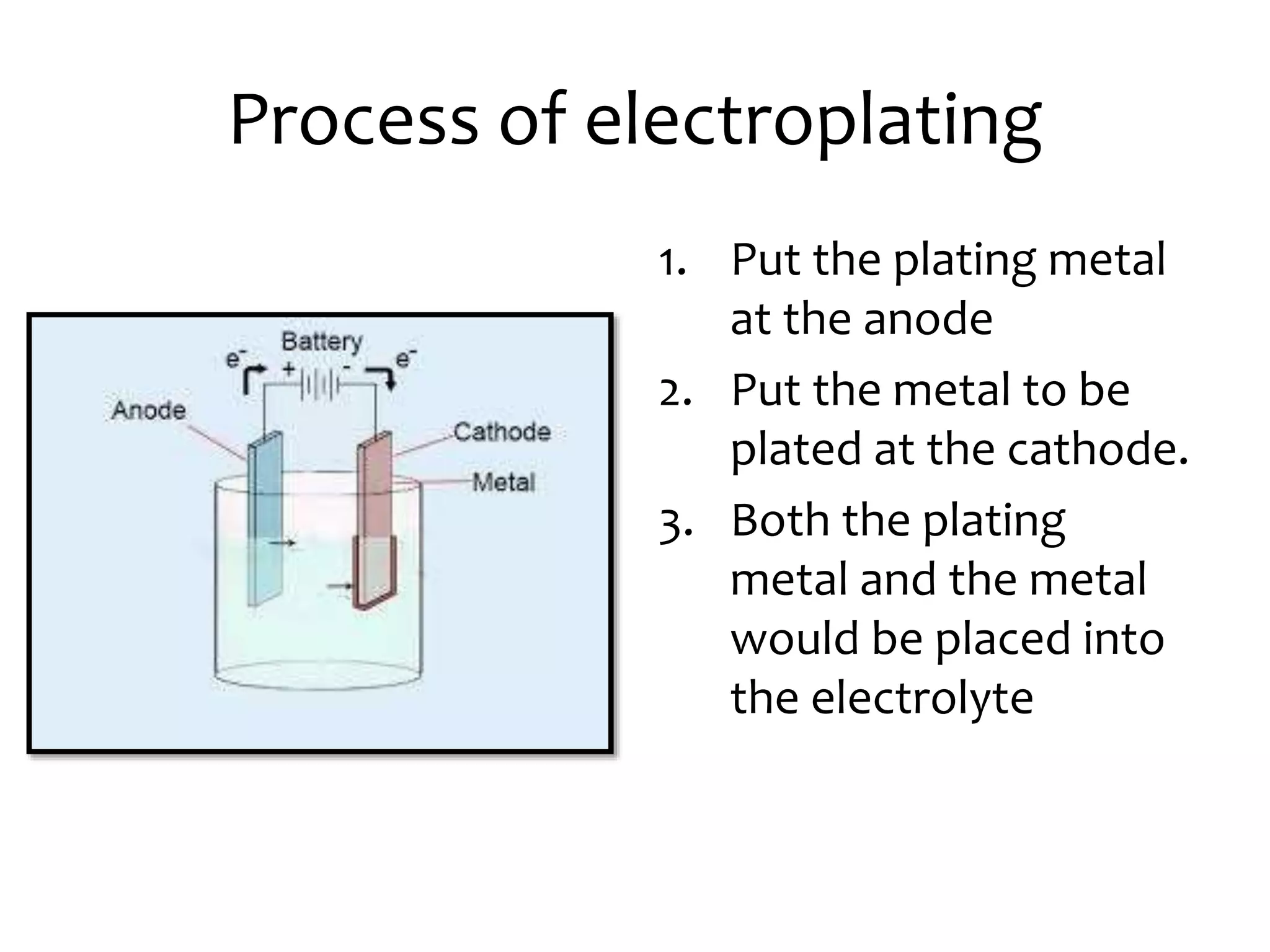



Acids and salts speed up the rate of rusting by increasing the concentration of ions, allowing more ion movement which causes iron to lose electrons faster and form iron oxide. Rusting can be prevented by applying a barrier like paint, oil, grease or electroplating between the metal and oxygen/water. Electroplating involves placing the plating metal as the anode and the metal to be plated as the cathode in an electrolyte solution. Common metals used for plating include tin, silver, nickel, chromium, zinc and gold.









