BIOMOLECULE PRESENTATION BY MUSKAN.pptx
- 1. BIOMOLECULE TOPIC:- ENZYME MUSKAN MSC MICROBIOLOGY
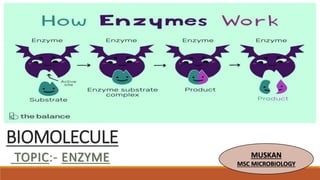
- 2. ENZYME Enzymes are the biological macromolecules which speed up the rate of biochemical reactions without undergoing any change. They are also called as biological catalysts. An enzyme is a highly selective catalyst that greatly accelerates both the rate and specificity of metabolic reactions. Always present in insoluble form, either be enzyme have tertiary structure. Nearly all enzymes are proteins, but all proteins are not enzymes. Enzyme catalyzed reactions usually take place under relatively mild conditions (temperatures well below 100oC, atmospheric pressure and neutral pH) as compared with the corresponding chemical reactions. Enzymes are highly specific with respect to the substrates on which they act and the products that they form. Enzyme activity can be regulated, varying in response to the concentration of substrates or other molecules. They function under strict conditions of temperature and pH in the body.
- 4. DICOVERY AND NOMENCULTURE AND CLASSIFICATION ◦ Firstly discovered of enzyme by( EDWARD BUCHNER) as by chance. ◦ Firstly zymes enzyme are coined by Louis pasture which is used in fermentation process. NOMENCULTURE: SUBSTRATE ACTED UPON BY THE ENZYME: ◦ Named the enzyme by adding the “ase” in the name of the substrate catalyzed, Eg:- proteins – proteinase. TYPE OF REACTION CATALYZED: ◦ The enzyme are named by adding the “ase” in the name of reaction. Eg:- Hydrolase – catalyzing hydrolysis. INTERNATIONAL UNION OF BIOCHEMISTRY (IUB) CLASSIFICATION: ◦ The chemical reaction catalyzed is he specific property which distinguishes one enzyme from another . ◦ In 1961, IUB used this creation as a basis for the classification and naming of enzyme. ◦ According to IUB enzyme give a( EC) NO, EC no have 4 digit then either e classify 6 major clasess and 13 sub classes. ◦ EC no. denoted by 2.1.6.4(2- belong to a class), (1- belong to a sub class),(6- sub division),(4- reaction catalyse)
- 5. class 1 - OXIDOREDUCTASES: Enzyme which bring about oxidation and reduction reactions between two subsrate. A++ B+++ A+++ B++ Eg: peroxidase, dehydrogenase,cytochrome oxidase. Class 2- TRANSFERASE: enzyme which catalyzed the transfer of group, AX B A BX Eg: Transaldolase, hexokinase, trans ketolase, Class 3- Hydrolase: breakdown of molecule with presence of water. Eg: 2sucrose water sucrase glucose fructose All digestive enzyme are in this catagery Class 4 – Lyase: breakdown of molecule but without presence of water. Eg: aldolase, carbonic anhydrase
- 6. class 5 - Isomerase: rearrangement of atoms or group . Eg: phosphohexos isomerase ( respiration reaction), Class 6 – Ligase: help in bonding formation . Eg: DNA ligase ( joint the replication fork ) , peacase. TRICK 1 . O 2. T 3. H 4. L 5. I 6. L
- 7. TYPE OF ENZYME ALL ARE PROTEIN Eg: trypsin > CONJUGATED ENZYME ARE FUNCTIONALLITY POSSIBLE that have non protenatious enzyme show the funcatinallity. COFACTOR EITHER BE ORGANIC AND INORGANIC FORM >LOOSELY BOUNDED WITH APO ENZYME > TIGHTLY BOUND WITH APOENZYME Eg: - RIBOFLAVIN Eg: - heam group SIMPLE ENZYME CONJUGATED ENZYME PROTEIN ENZYME ( APOENZYME) NON PROTEIN ENZYME (COFACTOR) HOLOENZYME ORGANIC PROSTHET- HIC GROUP
- 8. ZN+ , > Eg:- FAD+ NADH+ CLORIDE FOR ACTIVATION SALIVARY AMYLASE KARBOXIPEPTIDASES COFACTOR INORGANIC FORM METAL ION NON METAL ION
- 9. SIGNIFICANE OF ENZYMES In the absence of an enzyme, biochemical reactions hardly proceed at all, whereas in its presence the rate can be increased up to 107-fold. Thus, they are crucial for normal metabolism of living systems. Besides in the body, extracted and purified enzymes have many applications. Medical applications of enzymes include: To treat enzyme related disorders. To assist in metabolism To assist in drug delivery. To diagnose & detect diseases. In manufacture of medicines. Industrial applications of enzymes include: Amylase, lactases, cellulases are enzymes used to break complex sugars into simple sugars. Pectinase like enzymes which act on hard pectin is used in fruit juice manufacture. Lipase enzymes act on lipids to break them in fatty acids and glycerol. Lipases are used to remove stains of grease, oils, butter. Enzymes are used in detergents and washing soaps. Protease enzymes are used to remove stains of protein nature like blood, sweat etc.
- 10. MECHANISM OF ENZYMATIC ACTION Catalysis is the prime function of enzymes. Enzymes are powerful catalysts. The nature of catalysis taking place in the biological system is similar to that of non-biological catalysis. For any chemical reaction to occur, the reactants have to be in an activated state or transition state. Enzymes lower activation energy : The energy required by the reactants to undergo the reaction is known as activation energy. The reactants when heated attain the activation energy. The catalyst (or the enzyme in the biological system) reduces the activation energy and this causes the reaction to proceed at a lower temperature. Enzymes do not alter the equilibrium constants, they only enhance the velocity of the reaction. The role of catalyst or enzyme is comparable with a tunnel made in a mountain to reduce the barrier. The enzyme lowers energy barrier of reactants, thereby making the reaction go faster. The enzymes reduce the activation energy of the reactants in such a way that all the biological systems occur at body temperature (below 40°C).
- 11. Enzyme-substrate complex formation The prime requisite for enzyme catalysis is that the substrate (S) must combine with the enzyme (E) at the active site to form enzyme-substrate complex (ES) which ultimately results in the product formation (P). S E (E-S) (E-P) P+ E
- 12. Lock and key model or Fischer’s template theory This theory was proposed by a German biochemist, Emil Fischer. This is in fact the very first model proposed to explain an enzyme catalysed reaction. According to this model, the structure or conformation of the enzyme is rigid. The substrate fits to the binding site (now active site) just as a key fits into the proper lock or a hand into the proper glove. Thus the active site of an enzyme is a rigid and pre-shaped template where only a specific substrate can bind. This model does not give any scope for the flexible nature of enzymes, hence the model totally fails to explain many facts of enzymatic reactions, the most important being the effect of allosteric modulators.
- 13. INDUCED FIT HYPOTHESIS Koshland proposed this hypothesis in the year 1960. This hypothesis is actually quite different from the previous hypothesis. It states that the active site of the enzyme is flexible in shape and can change its shape according to the nature of the substrate, which means that it can form its active site complementary to the substrate. It is easy to understand how a hand induces a change in the glove, that is, the same way an active site induces a change in the chemical substrate. The substrate gets into the active site of the enzyme. According to this, the structure of the active site of an enzyme is flexible. There are two types of groups that are present in the active site of the enzyme. One is a buttressing group and the other is a catalytic group. The buttressing group helps in supporting the substrate, whereas the catalytic group helps to explain the mechanism of enzyme catalyses. When the buttressing group comes in contact with the substrate, changes take place in the active site and these changes help to bring the catalytic group opposite to the substrate bonds that are needed to be broken. The above two models help us deeply understand and describe the mechanism of enzyme action.
- 15. SUBSTRATE STRAIN THEORY In this model, the substrate is strained due to the induced conformation change in the enzyme. It is also possible that when a substrate binds to the preformed active site, the enzyme induces a strain to the substrate. The strained substrate leads to the formation of product. The concept of substrate strain explains the role of enzyme in increasing the rate of reaction. In fact, a combination of the induced fit model with the substrate strain is considered to be operative in the enzymatic action.
- 16. FACTORS AFFECTING MECHANISM OF ENZYME ACTION 1. Concentration of enzyme: Velocity of a reaction is directly proportional to the enzyme concentration. This property is used for determining the activities of serum enzymes during the diagnosis of diseases. 3. Temperature: Raising temperature generally speeds up a reaction, and lowering temperature slows down a reaction. However, extreme high temperatures can cause an enzyme to lose its shape (denature) and stop working.
- 17. 2. Concentration of substrate:
- 18. In the presence of a given amount of enzyme, the rate of enzymatic reaction increases as the substrate concentration increases until a limiting rate is reached, after which further increase in the substrate concentration produces no significant change in the reaction rate. At this point, so much substrate is present that essentially all of the enzyme active sites have substrate bound to them. In other words, the enzyme molecules are saturated with substrate. The excess substrate molecules cannot react until the substrate already bound to the enzymes has reacted and been released (or been released without reacting). The velocity of the reaction increases with an increase in the concentration of substrate initially. It reaches the peak value (Vmax) and there is no further increase after that. At this point, all the active sites of the enzyme get saturated with the substrate and there are no sites left for binding with the substrate. Enzyme activity is also affected by regulators such as competitive inhibitor. The competitive inhibitor has structural similarity with the substrate molecule and competes for the binding site present on enzymes, e.g. malonate inhibits succinate dehydrogenase due to structural similarities with succinate, which is the substrate for succinate dehydrogenase enzyme.
- 19. .EFFECT OF TEMPERATURE AND pH: Enzymes get denatured at extreme temperatures and pH and lose their ability to bind to substrates. Each enzyme has a specific optimum temperature and pH at which it shows the maximum activity, e.g. optimum pH for pepsin is 1.8, whereas for salivary amylase it is 6.8. There is a decline in its activity both above and below the optimum value.
- 20. The protein nature of the enzymes makes them extremely sensitive to thermal changes. Enzyme activity occurs within a narrow range of temperatures compared to ordinary chemical reactions. As you have seen, each enzyme has a certain temperature at which it is more active. This point is called the optimal temperature, which ranges between 37 to 40C°. The enzyme activity gradually lowers as the temperature rises more than the optimal temperature until it reaches a certain temperature at which the enzyme activity stops completely due to the change of its natural composition. On the other hand, if the temperature lowers below the optimal temperature, the enzyme activity lowers until the enzyme reaches a minimum temperature at which the enzyme activity is the least. The enzyme activity stops completely at 0C°, but if the temperature rises again, then the enzyme gets reactivated once more.
- 21. 4. Effect of pH The potential of hydrogen (pH) is the best measurement for determining the concentration of hydrogen ion (H+)in a solution. It also determines whether the liquid is acidic, basic or neutral. Generally, all liquids with a pH below 7 are called acids, whereas liquids with a pH above 7 are called bases or alkalines. Liquids with pH 7 are neutral and equal the acidity of pure water at 25 C°. You can determine pH of any solution using the pH indicators. pH Indicators Enzymes are protein substances that contain acidic carboxylic groups (COOH–) and basic amino groups (NH2). So, the enzymes are affected by changing the pH value. Each enzyme has a pH value that it works at with maximum efficiency called the optimal pH. If the pH is lower or higher than the optimal pH, the enzyme activity decreases until it stops working. For example, pepsin works at a low pH, i.e, it is highly acidic, while trypsin works at a high pH, i.e, it is basic. Most enzymes work at neutral pH 7.4.
- 23. THANK YOU .