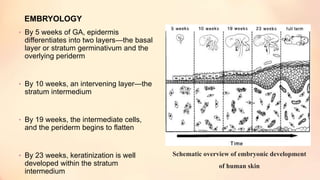
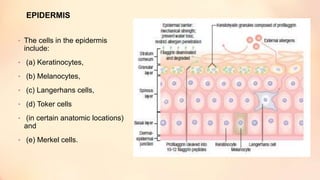
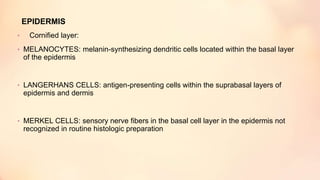
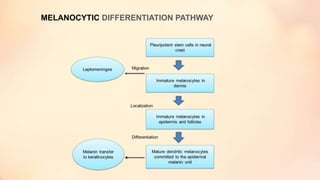
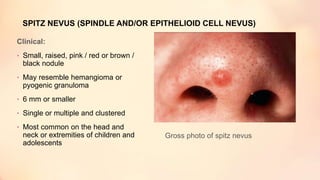
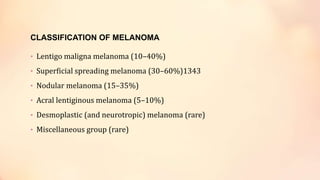
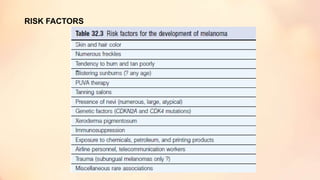
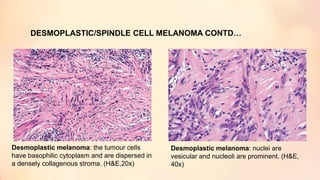
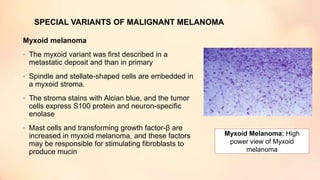
The document outlines the embryological development and histological structure of melanocytic lesions, describing the layers of skin and the types of cells present, including keratinocytes, melanocytes, and Langerhans cells. It details various forms of melanocytic nevi, their clinical features, and histological classifications, alongside the pathogenesis of lesions like solar lentigo and lentiginous melanoma. Additionally, it discusses the maturation of melanin and factors influencing melanocyte activity, emphasizing sun exposure as a significant risk factor for melanoma.













































































































![REFERENCES
• Weedon D. Weedon's skin pathology. 3rd ed. Churchill
Livingstone: Elsevier; 2010.
• Humphrey PA, Dehner LP, Pfeifer JD. Washington Manual of
Surgical Pathology. [S.l.]: Wolters Kluwer Health; 2015.
• Mills SE. Histology for Pathologists. 3rd ed. Lippincott Williams
and Wilkins; 2007.
• McKee P, Calonje E, Brenn T, Lazar A. McKee's pathology of the
skin. [Edinburgh]: Elsevier Saunders; 2012.](https://image.slidesharecdn.com/melanocyticlesions-newbackground-180324165144/85/Melanocytic-lesions-Pathology-119-320.jpg)
