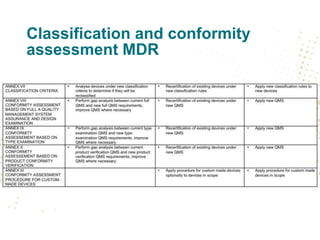
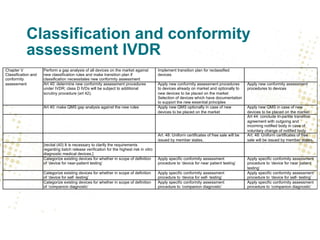
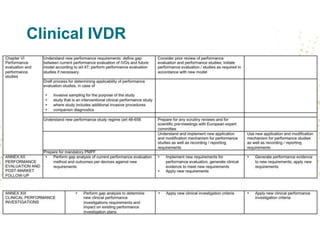
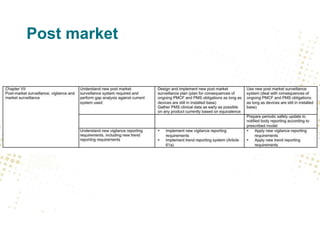
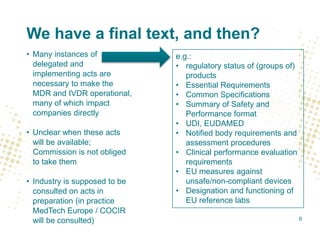
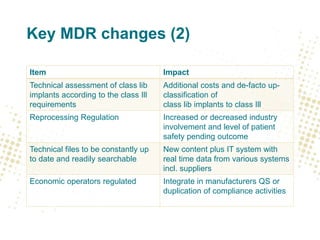
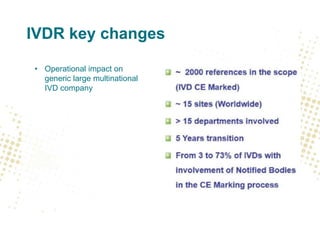
The document discusses key changes and requirements regarding the EU Medical Devices Regulation (MDR) and In Vitro Diagnostics Regulation (IVDR) and the European database on medical devices (Eudamed). Some of the main points discussed include:
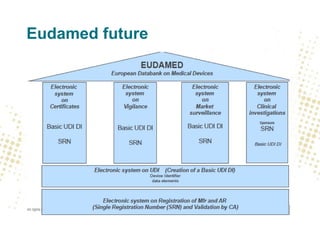
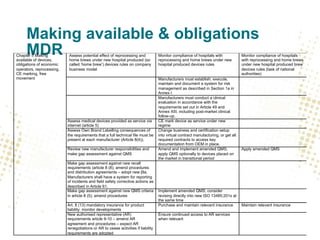
- Eudamed will contain integrated electronic systems for European UDI, registration of devices and economic operators, scrutiny applications, certificates, clinical investigations, vigilance, and market surveillance.
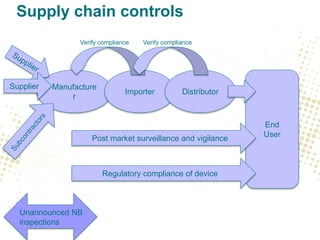
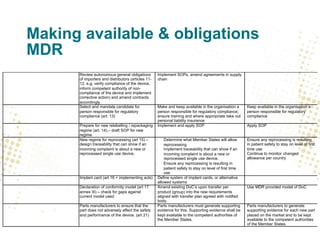
- Traceability requirements will require manufacturers, distributors, and importers to cooperate to achieve appropriate traceability levels and identify economic operators in the supply chain.
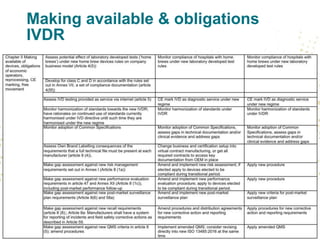
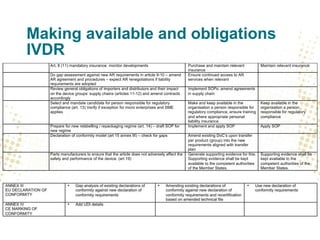
- Unique Device Identification (UDI) must be assigned and placed on labels and packaging. Registrations of devices and economic












![The Eudamed “cathedral”
• Will Eudamed realistically be
ready to support
• all these functions
• in time?
“Who knows where the road
may lead us, only the fool
would say
Who knows if we'll meet along
the way
Follow the brightest star as far
as the brave may dare
What will we find when we get
there”
[Alan Parsons Project – La
Sagrada Familia]
OR](https://image.slidesharecdn.com/advamedmdrivdrupdate-160525212739/85/Advamed-MDR-IVDR-update-22-320.jpg)