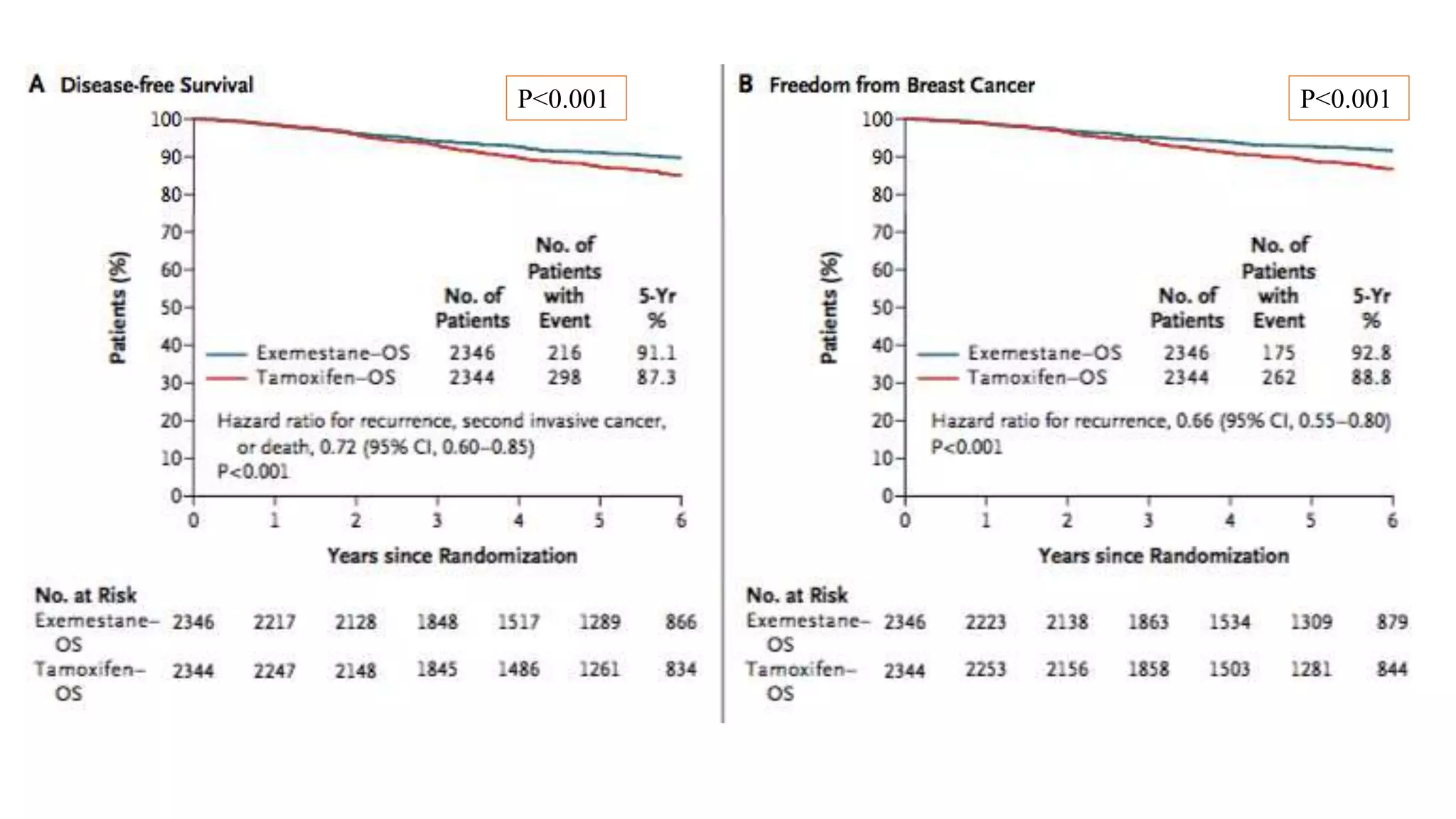
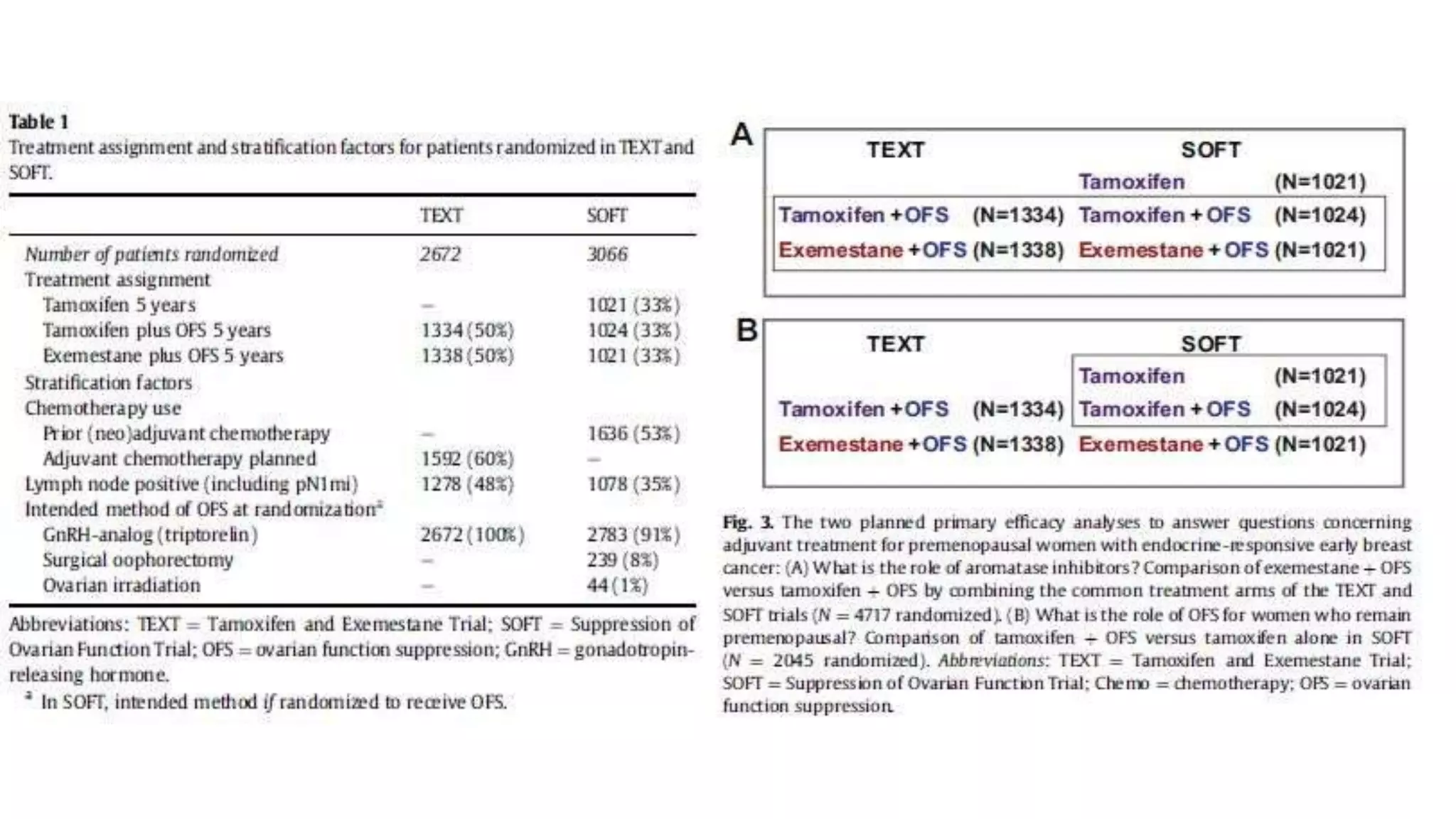
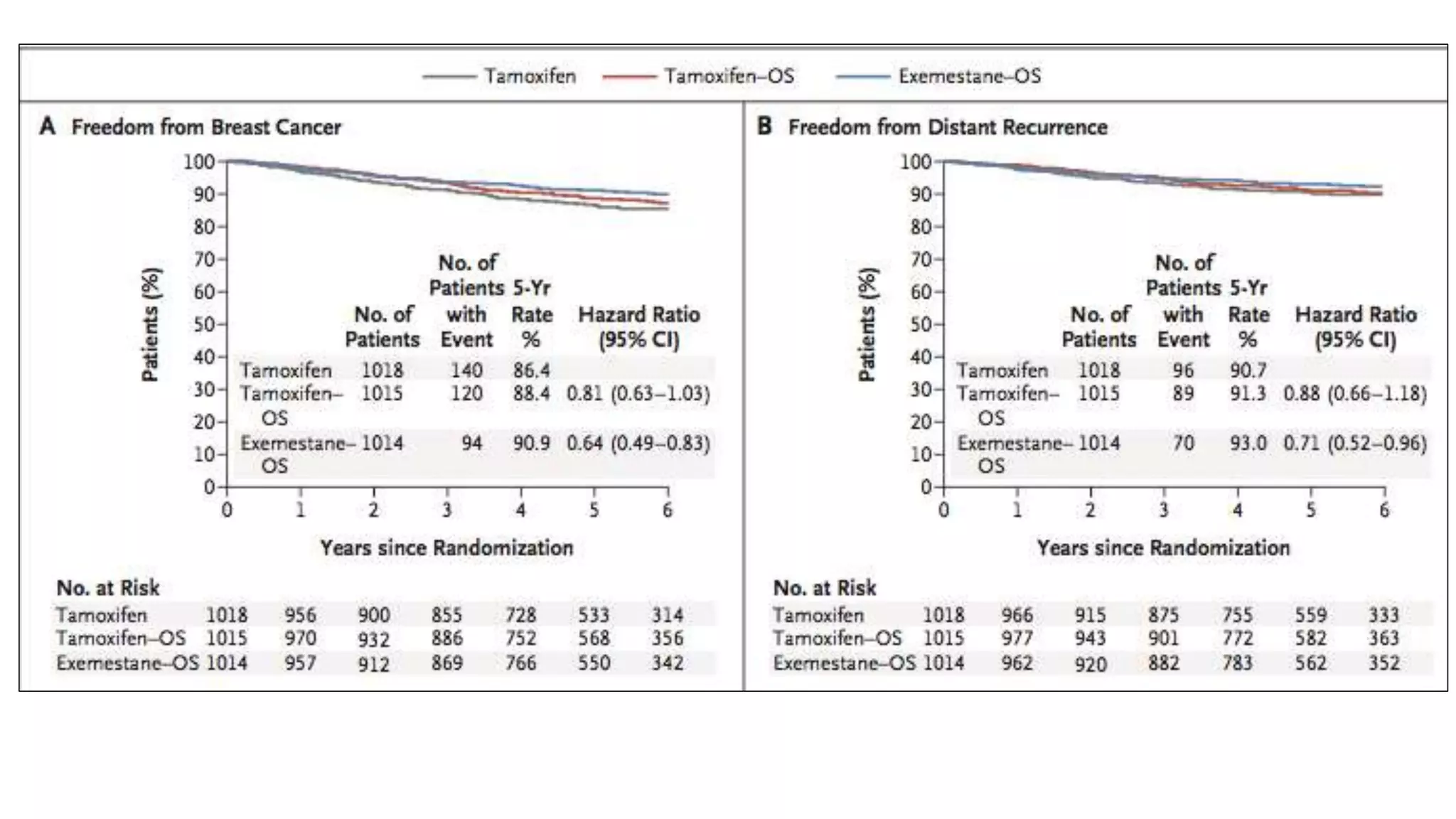
The SOFT trial investigated the role of ovarian function suppression (OFS) and aromatase inhibitors (AIs) in premenopausal breast cancer patients. It found that adding OFS to tamoxifen (TAM) improved 5-year disease-free survival from 84.7% to 86.6%, though the difference was not statistically significant. The SOFT+TEXT analysis found that substituting exemestane for TAM when combined with OFS improved 5-year disease-free survival from 87.3% to 91.1%, with a statistically significant difference. For patients who received chemotherapy, exemestane+OFS provided an absolute 5-year breast cancer-free rate improvement of 3.


















![Summary – SOFT Trial
SOFT Tamoxifen-OFS Tamoxifen P value
5-yr DFS 86.6% 84.7% 0.10
5-yr OS 96.7% 95.1% 0.13
5-yr BC freedom rate 88.4% 86.4% 0.09
• In the low risk subpopulation of patients who did not receive chemotherapy
(predominantly women >40 years, with small, node-negative [N-] tumors of low to
intermediate grade), >95% remained free from BC and without distant recurrences at 5
years with TAM alone. In this cohort of patients TAM alone is very effective and can still
be considered the standard of care.](https://image.slidesharecdn.com/jcsofttext1-161228191317/75/SOFT-TEXT-Trials-27-2048.jpg)