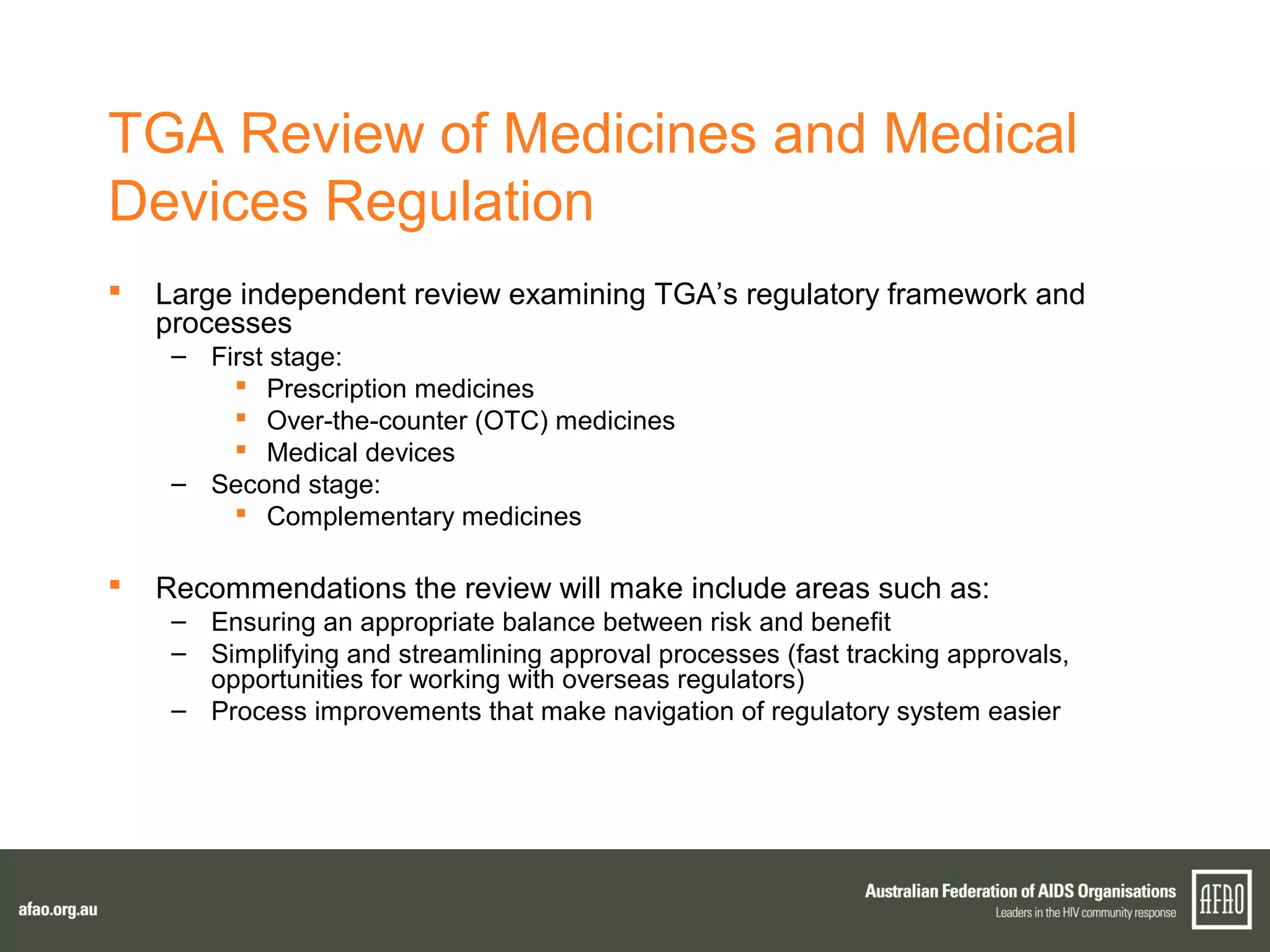
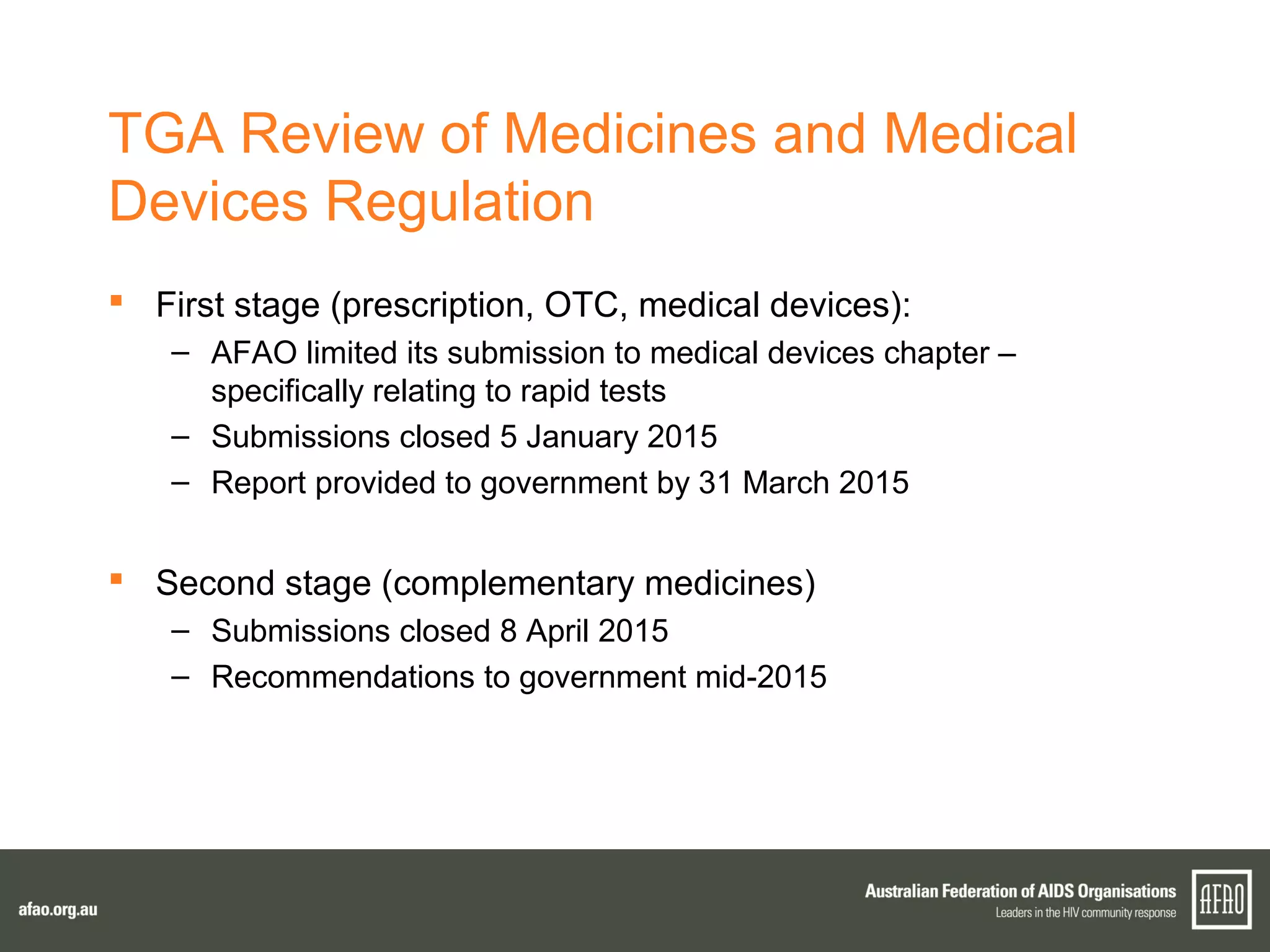
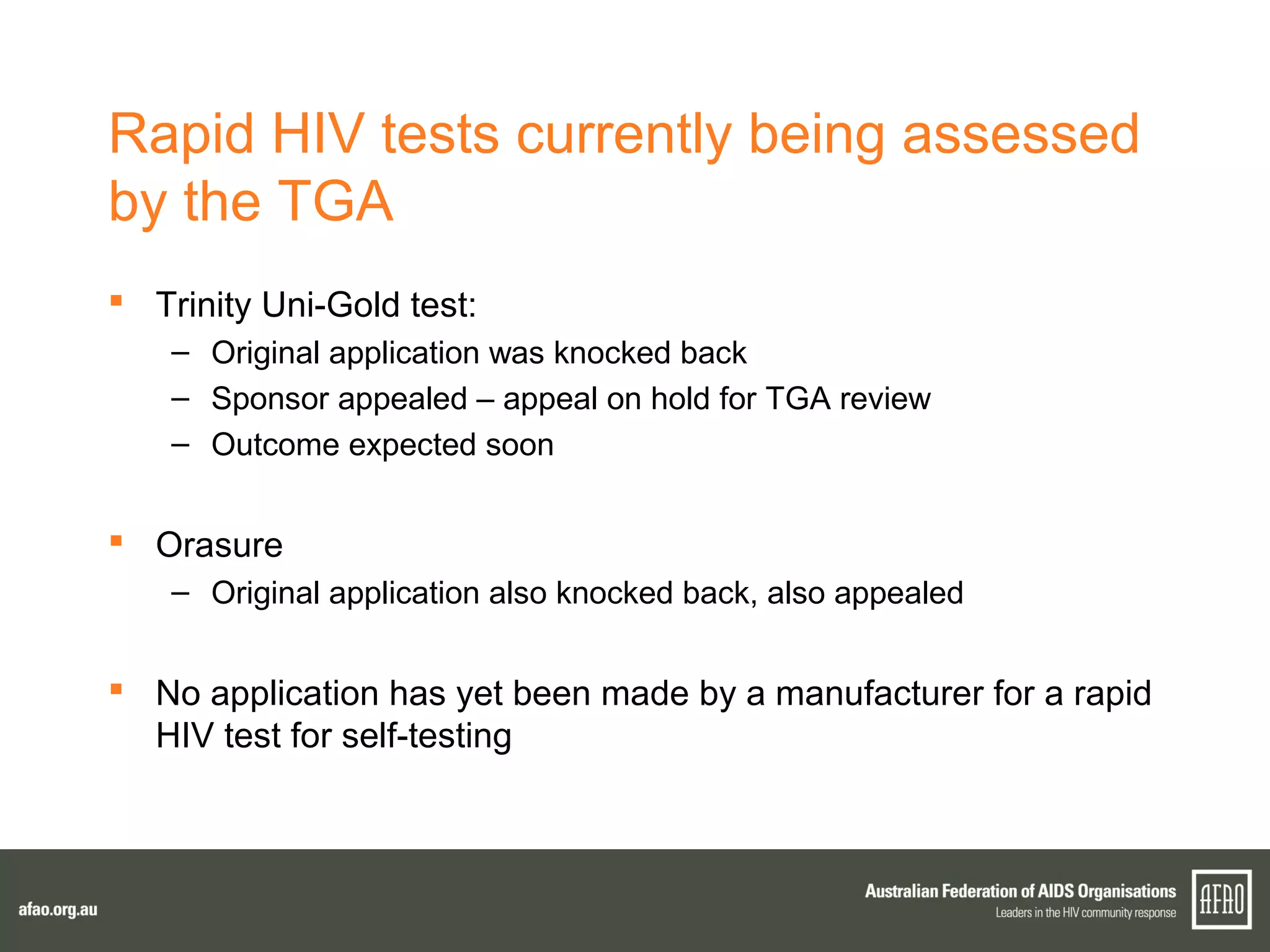
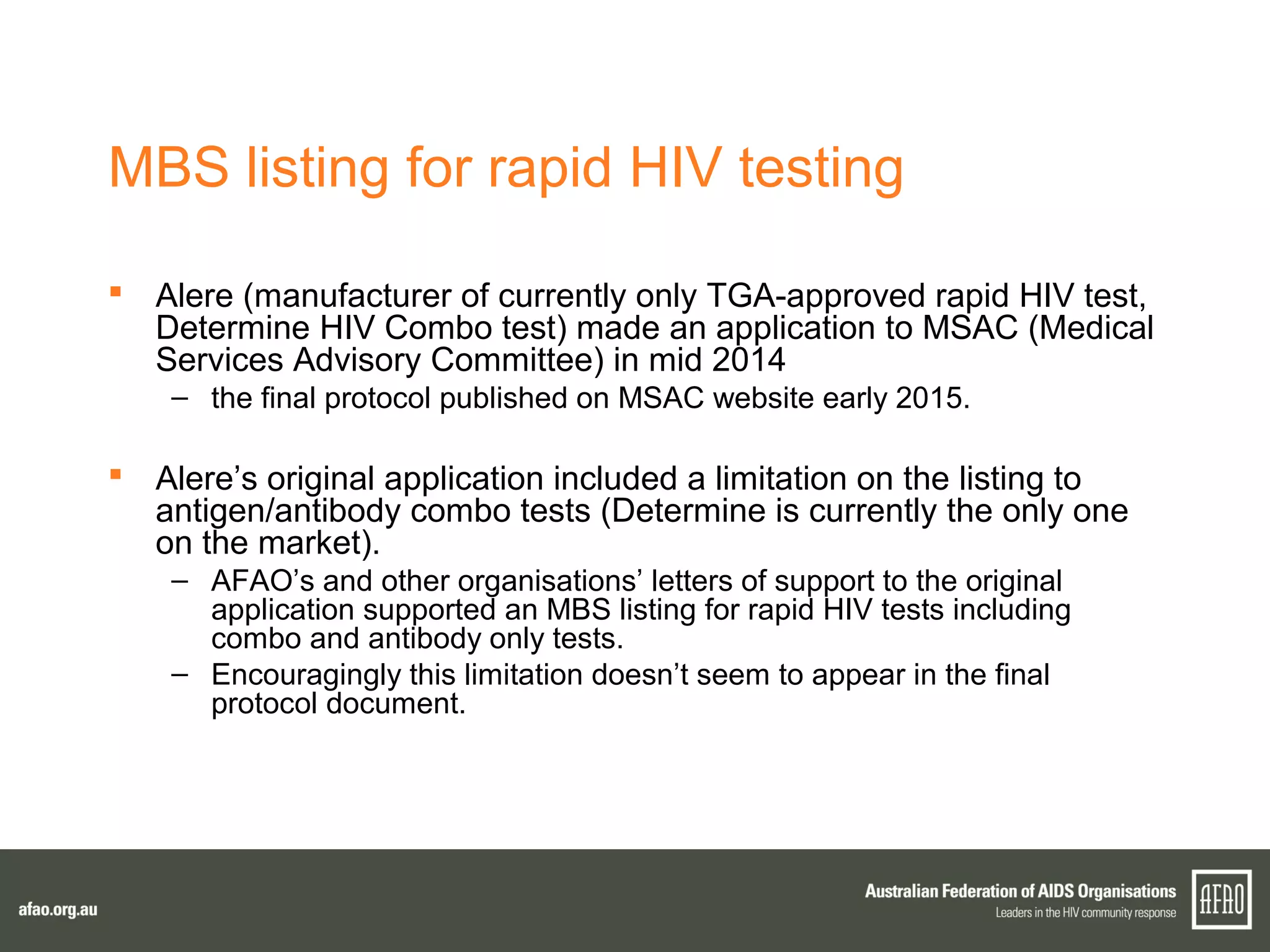
The document discusses various regulatory updates from the TGA regarding medicines and devices, focusing on HIV tests, including rapid and self-testing formats. It outlines submissions and recommendations from the AFAO concerning the medical device regulation review, as well as updates on the MBS listing process for rapid HIV testing. Furthermore, the national HIV testing policy is under revision to incorporate amendments, and expert groups are working towards finalizing policies by mid-2015.