Chapter6卤代烷亲核取代
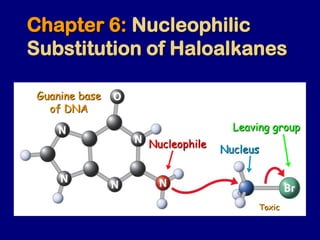
- 1. Chapter 6: Nucleophilic Substitution of Haloalkanes Guanine base of DNA Leaving group Nucleus Nucleophile Toxic
- 2. Haloalkanes Names: Halo-, as a substituent 1-Chlorobutane (1S,2R)-1-Bromo-2- fluorocyclohexane 2-Iodo-2- methylbutane C + - X Cl 4 3 2 1 F BrR S I CH3
- 3. The C-X Bond is Polarized ClCH3 + - Electrophilic
- 5. Trends are a function of dipole- dipole and London forces Electronegativity (4.0) (3.2) (3.0) (2.7)
- 6. Nucleophilic Substitution: General Color scheme: Nu, E, L, and curved arrows: e-Flow C XNu + C XNu - + Nucleophile (Nu) Leaving group (L) Electrophile (E) -
- 7. Remember: Acid-base reactions B + H A B H + A - - conjugate acid base - B = Nu - when H is attacked, we call it base B. When C (or other nuclei) attacked, we call it nucleophile Nu.
- 8. Note: no (simple) H º calculations possible on ionic reactions; bond strengths refer to homolytic, not heterolytic, dissociation.
- 9. Note: Tertiary halides are notably absent from this list.
- 10. Mechanism In general: how do we study it ? 1. Kinetics 2. Stereochemistry 3. Modify substituents: look for electronic and steric effects 4. Isotope effects: Usually H/D DHº C--H < C—D 5. Modify reagents/subtrates: Nu, E, L, solvent
- 11. Kinetics For HO + CH3 Cl CH3OH + Cl -- Rate = k [CH3Cl][ OH] 2nd order Points to bimolecular mechanism (TS) Hence name: SN2 bimolecular, nucleophilic substitution -
- 12. [HO···CH3Cl]‡ ? CH3Cl CH3OH E What is TS structure? We can look at stereochemistry. Two extreme approaches of Nu : C X Back Front + Cl- + -OH Transition State ―
- 13. Frontside attack: Retention of configuration. Backside attack: Inversion of configuration. Which one is it? C X chiral * Test: Use enantiomerically pure
- 16. C Br S H H3C CH3CH2 + - I C BrI - + H CH3 CH2CH3 R Result: Inversion (no S -product)
- 18. Chemical Consequences of Inversion 1. Retention: By double inversion C CH3 R H Br C CH3 R H SHCI CH3 R H I - Br - I - H S - + - - + 2. Inversion does not necessarily mean: R S CH3CH2O + C CH3S H H3C Br C X CH3CH2O -+ SCH3 H CH3 S c b ab c a- S
- 19. 3. Diastereoisomerization CH3 Br H CH3 CH3CH2 H CH3 H H CH3 CH3CH2 II - Br R R R S - - Br H H3C H SS H CN H3C H RSCN - Br - - CH3 Br R S I - CH3 R R I Cis Trans - Br-
- 20. Leaving Group Ability “L” (kinetic parameter) C LNu + B + H A - What makes a good L- (A-) ? Remember from the discussion on acidity: 1. Ability to accommodate e-pair (charge) : e-Negativity + resonance 2. Size of the orbital describing the e-pair. 3. Indirectly: Bond strength C—L (H—A)
- 21. F < Cl < Br < I Increasing, going down periodic table (PT). HF HCl HBr HI pKa 3.2 -2.2 -4.7 -5.2 DHº 135 103 87 71 Why? Because: Goes down in PT And: Orbital size increases from 2p to 3p to 4p… As noted earlier: This trend is opposite that expected on the basis of electronegativity (goes down in PT). Same trend as HX: - - - - Halides as L
- 22. pKa 50 35 15.7 3.2 DHº 105 107 119 135 increases In practice: only F is a reasonable leaving group in this row. Hydroxide can be, in special cases. - CH3< NH2< OH< F : Electronegativity wins ! decreases, but And: Size of orbital decreases. R L or H A: Along a row of PT: L increases to the right (same trend as acidity)
- 23. For example, for same leaving atom, e.g., Generally: L increases to the right and down PT. But, superimposed on these trends: Resonance. pKa : 15.5 4.7 -1.2 - - - O O O - CH3O < CH3CO < CH3S O RO : (of acid)
- 24. Neutral L are good: relatively nonbasic 1. Protonated alcohols: L = H2O Use ROH plus HBr, or HI, or H2SO4 2. Diazonium ions: L = N2 , a superleaving group R OH + H R O H H Nu-+ R Nu + H2O R N N + Nu R Nu + N N -+ +
- 25. For practical purposes: Here is where L- ability ends (going down PT)
- 26. Nucleophilicity “Nu” (kinetic parameter) Affected by charge, basicity, solvent, polarizability, sterics. 1. Charge (for same atom): The more charged, the more nucleophilic HO > H2O ; H2N > H3N ; SO4 > ROSO3 - - 2- -
- 27. 2. Basicity: Decreases to the right in PT, so does Nu: H3N > H2O ; H2N > HO ; HO > F - - - - Comparison of neutral and charged Nu: (See pKa table) H2N > HO > H3N > F > H2O - - - As expected: Trend opposite L ability
- 28. Protic solvents have acidic H ; e.g., RO H or N H. They surround charged Nu , using hydrogen bonds: + +- +- - - R Down the PT: Murky! Basicity goes down, hence Nu should too (opposite L). But not true: Nu increases! The reason: Solvent effects and polarizability (deformability of orbital of ) have a strong influence. For charged Nu- : Nu Nu H OR
- 29. Protic Solvents: Fluoride is a Worse Nucleophile Than Iodide Hydrogen bonds - - - - - + + + + Solvent shell increases “size” of Nu , hence Nu increases going down PT. -
- 30. For uncharged Nu , same trend, but now due to polarizability H2O < H2S < H2Se (CH3)3N < (CH3)3P Polarizability operates also for charged Nu, which already benefit from lesser solvation: especially fast.
- 31. Increasing Polarizability Improves Nucleophilicity More polarizable Less polarizable
- 32. The Story Changes In Polar Aprotic Solvents, Which • dissolve salts • do not form H bonds • enable formation of “naked” anions cause huge rate increases with all Nu follow trends in basicity
- 34. Review: Range of Nucleophilicities • charge • basicity • polarizability • H-bonding Depends on:
- 35. Summary Trends in the Periodic Table L Nu- “Naked” anions, aprotic solvents or Protic solvent, polarizability e-Negativity > DHº and orbital size e-Negativity < DHº and orbital size
- 36. Steric Effects Sterics for L: Larger = better Sterics for Nu: Larger = worse, e.g., CH3O > (CH3)3CO - - Sterics around E are the most significant.
- 37. Sterics around electrophilic C L R Br + I R I + Br relative rates CH3 CH3CH2 (CH3)2CH (CH3)3C 145 1 0.078 0 Mechanism changes CH3CH2 CH3CH2CH2 (CH3)2CHCH2 (CH3)3CCH2 1 0.8 0.03 slow! 10-5 - - α : β : Alpha versus beta branching:
- 40. Walba DireStr