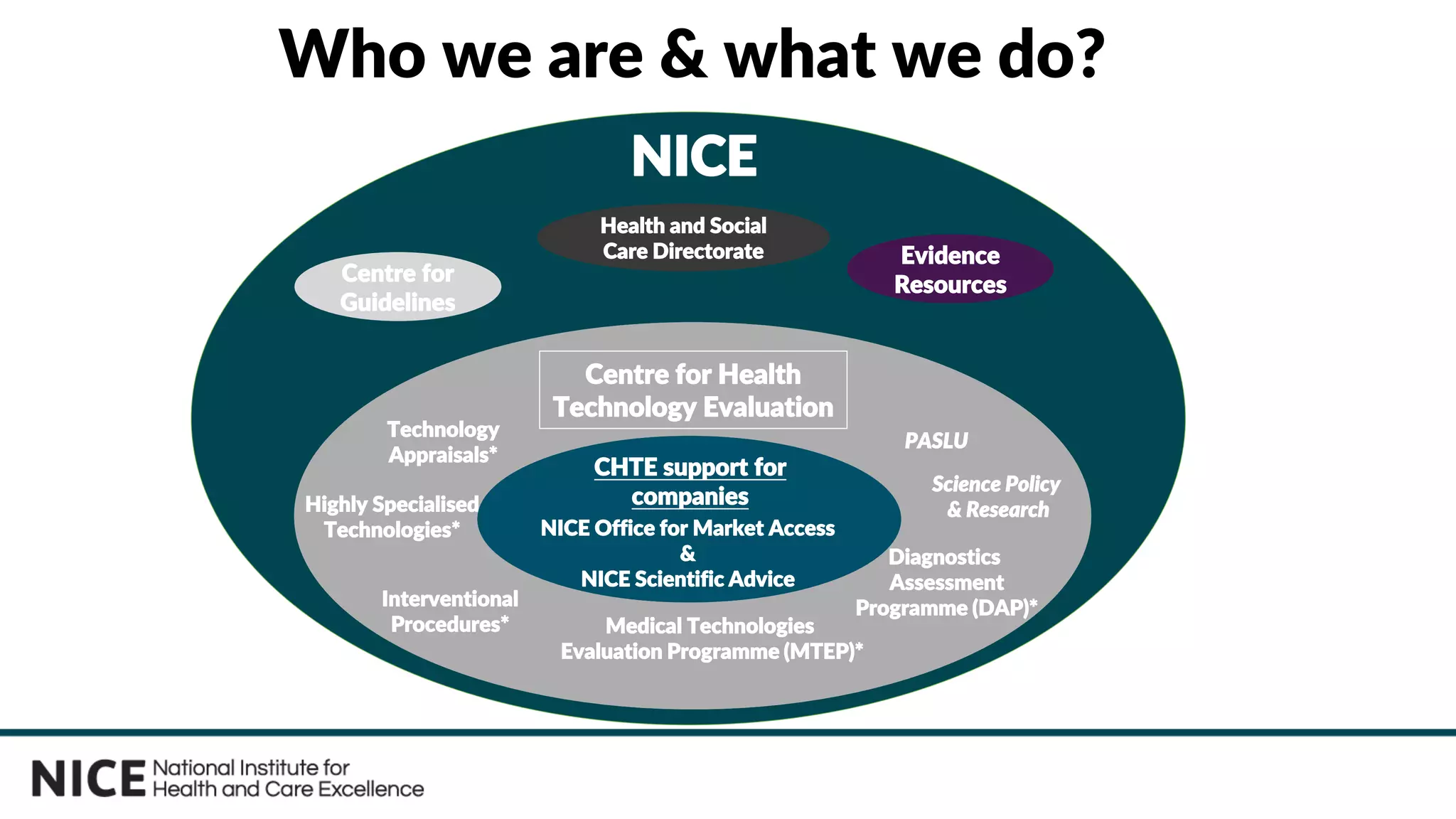
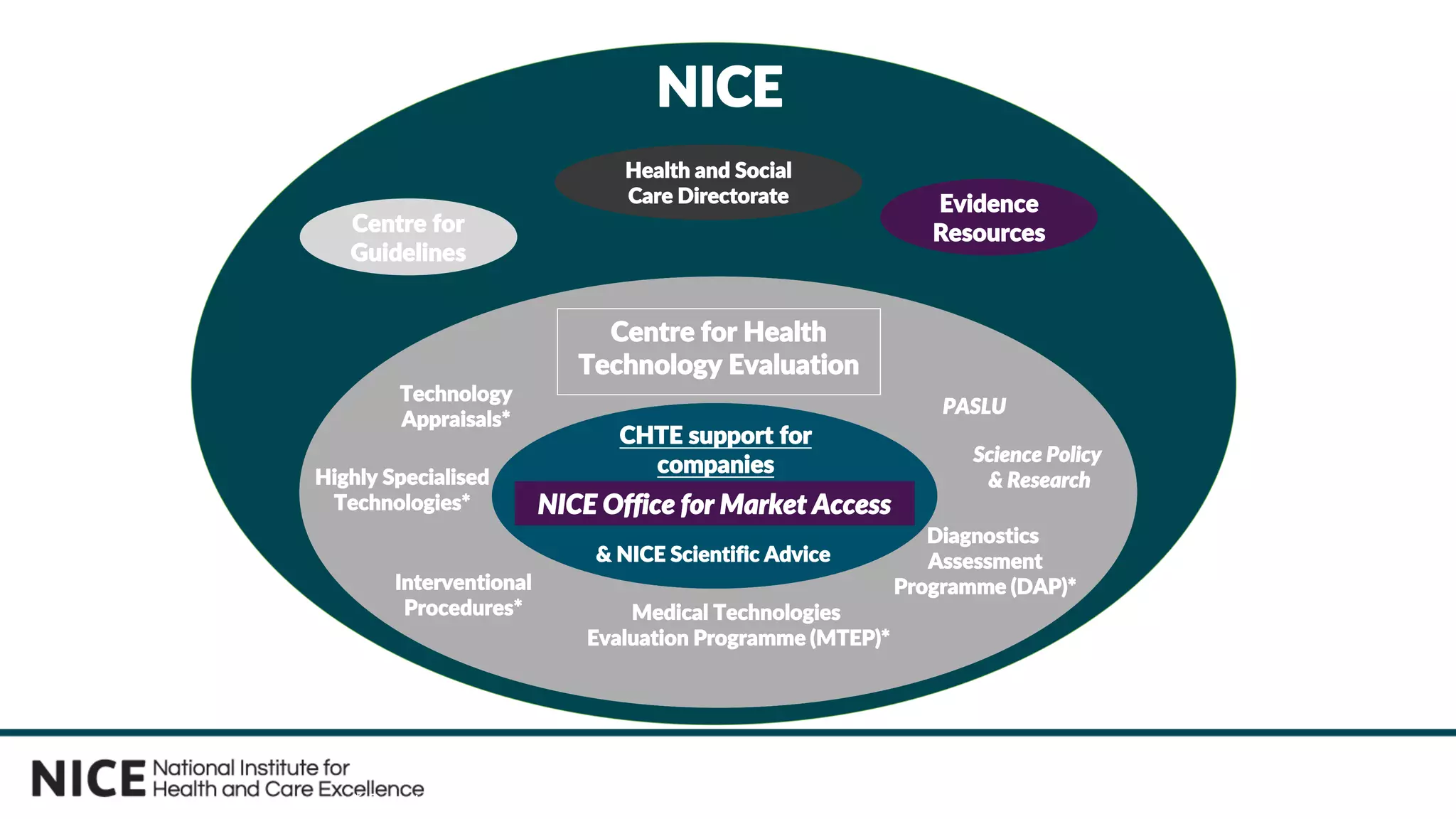
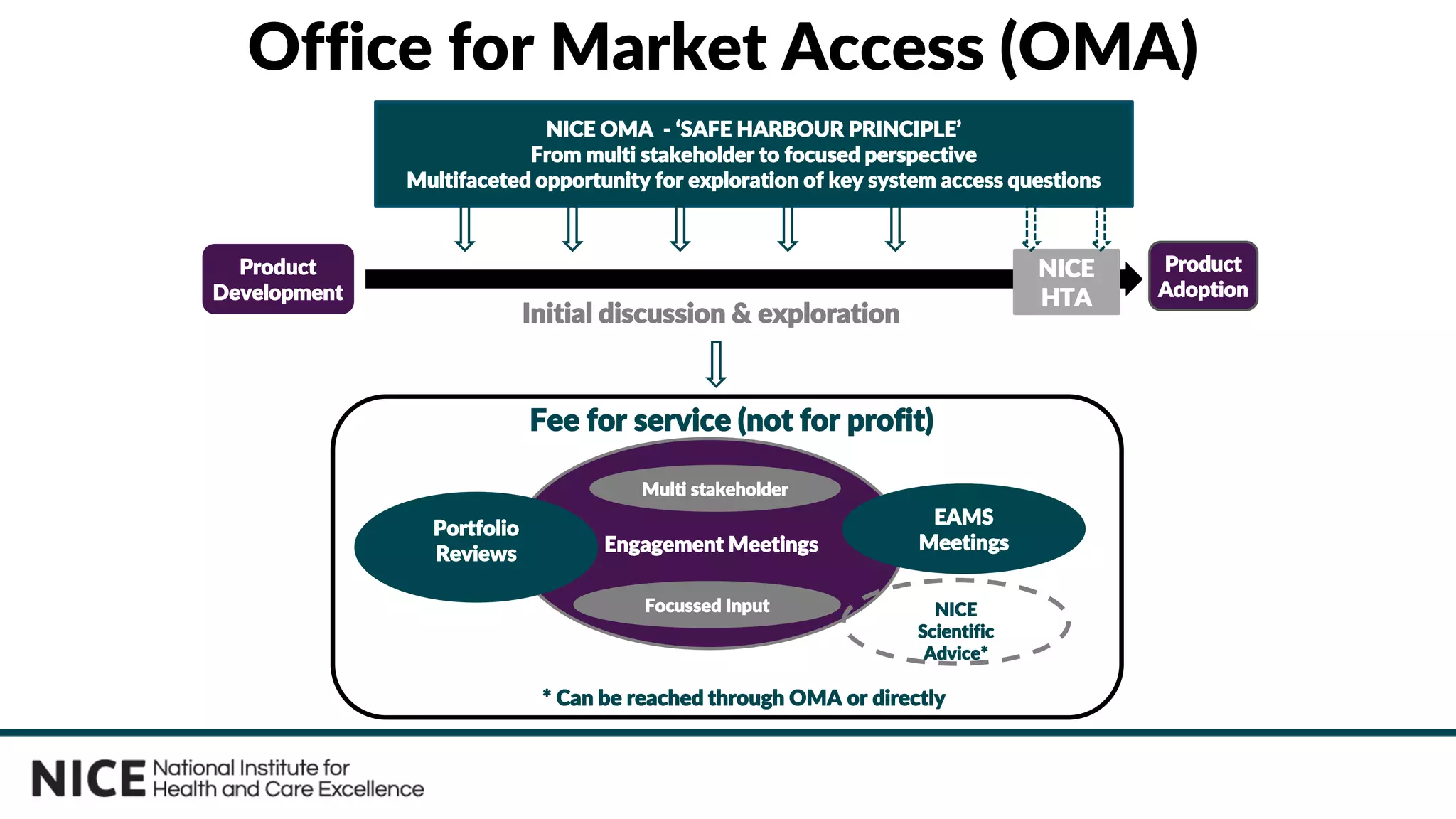
The document outlines the role and evolution of the National Institute for Health and Care Excellence (NICE) in the UK's healthcare system, emphasizing its commitment to evidence-based guidance and equitable access to treatments. It discusses the establishment of the Office for Market Access (OMA) to facilitate engagement with the life sciences sector and streamline the path from product development to adoption. The document highlights the importance of early engagement with NICE to understand its evaluation processes and improve access to innovative therapies.