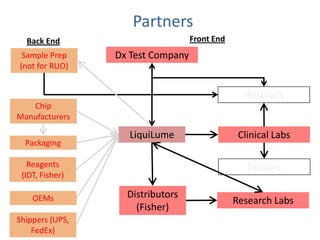
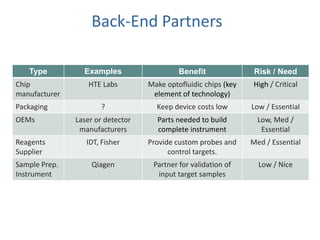
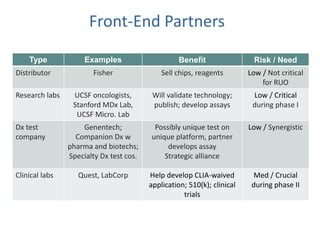
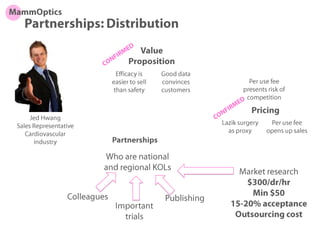
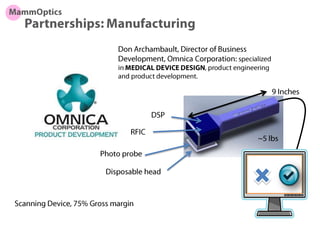
This document discusses partners and suppliers for life science startups. It identifies key types of partners including manufacturing, technology, channel/sales and marketing, and strategic partners. Manufacturing partners help with building, components, and outsourced functions. Technology partners contribute core technologies. Channel partners aid in sales and marketing. Strategic partners provide benefits like marketing support, intellectual property, or complementary products. The document also discusses front-end partners like distributors and research labs, and back-end partners such as chip manufacturers, packaging companies, and reagent suppliers. Maintaining valuable partnerships that evolve over time is important for startups.