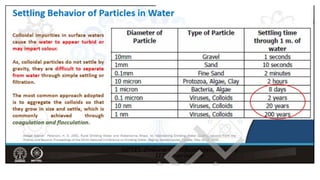
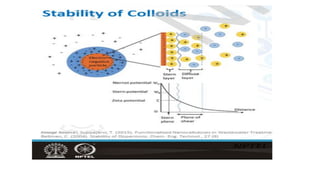
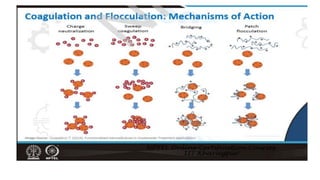
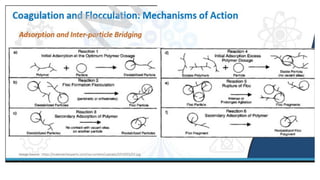
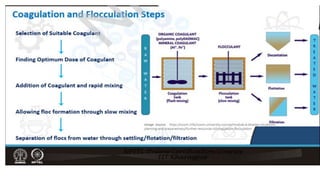
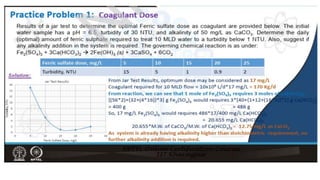
Coagulation and flocculation are two steps in water treatment that involve chemical and physical conditioning of water. Coagulation uses chemicals to reduce repulsion between particles, allowing them to come together. Flocculation then brings these destabilized particles together through slow mixing so they aggregate into flocs large enough to settle by gravity. Coagulant aids can help improve the coagulation process in waters where coagulants alone do not produce satisfactory flocs, and help coagulation work over a broader pH range. Proper coagulant dosing is important - overdosing increases costs and public health risks while underdosing reduces contaminant removal effectiveness.