1) Botijos and canteens with cloth covers cool water through evaporation. As water molecules gain energy and evaporate from the surface, they carry away heat in the form of latent heat of vaporization.
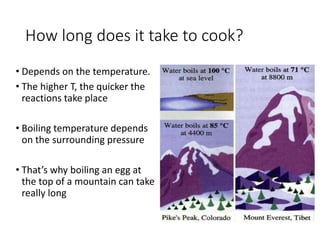
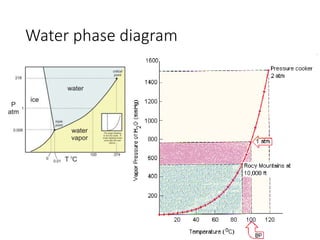
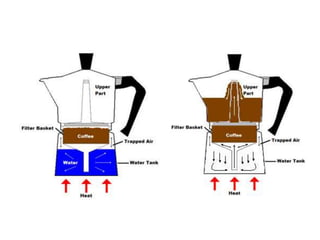
2) Pressure cookers cook food faster by raising the boiling point of water. At higher pressures like 2 atm, water boils at 120C rather than 100C, allowing chemical reactions in foods to occur more quickly.
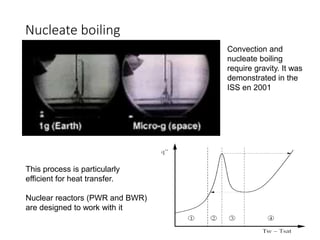
3) The sound of a boiling kettle comes from the process of nucleate boiling, where vapor bubbles form on imperfections in the kettle surface and rapidly grow and collapse, creating an audible noise.