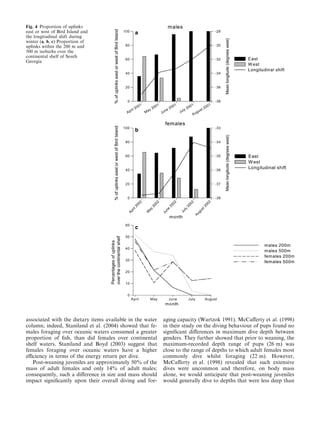
Male Antarctic fur seal pups foraged significantly farther from their birth site at South Georgia than female pups. Over the course of winter, both male and female pups' locations shifted eastward and moved farther from the continental shelf, possibly to exploit different prey in upper water columns. The study tracked 10 pups (5 males in 2001, 5 females in 2002) using satellite tags to analyze their at-sea distribution in relation to sex and environmental conditions between years.