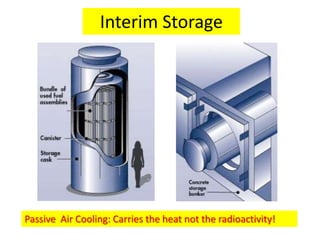
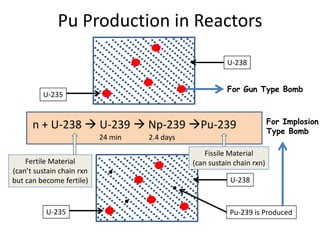
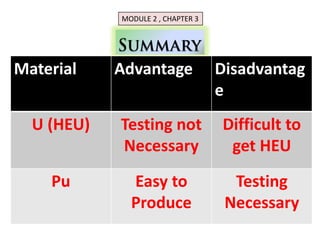
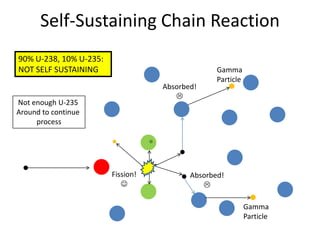
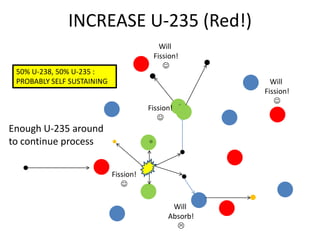
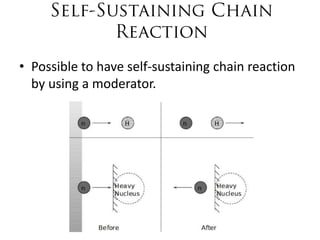
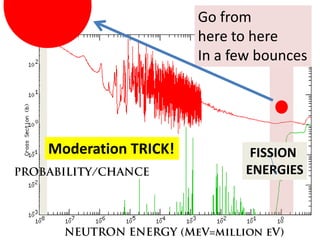
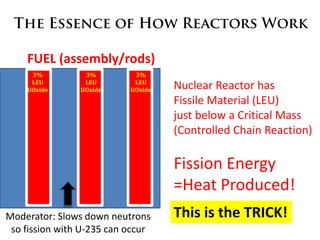
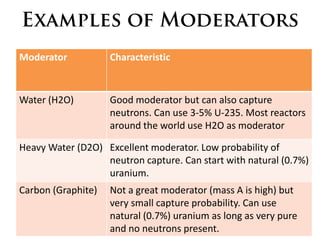
1. The document covers fundamentals of nuclear physics including radioactive decay, isotopes, induced and spontaneous fission, critical mass, and uncontrolled nuclear chain reactions.
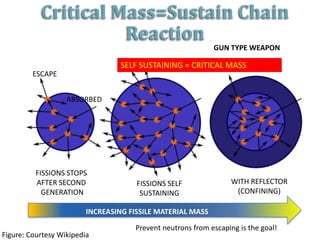
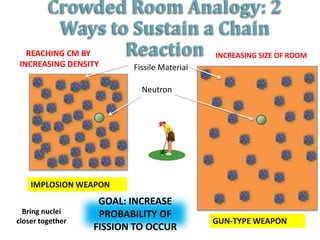
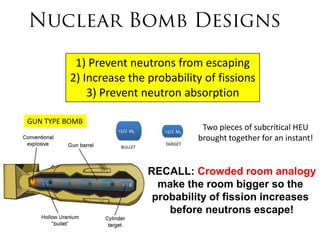
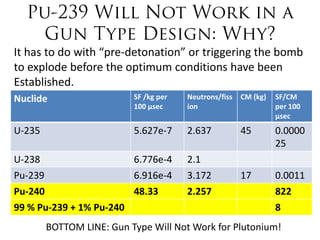
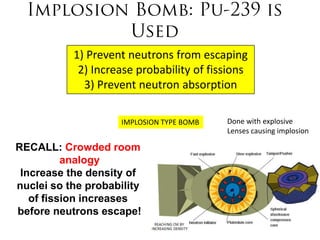
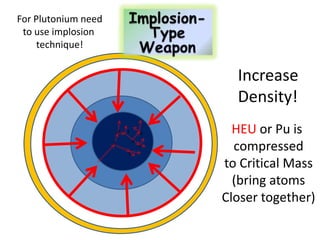
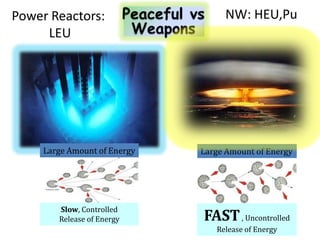
2. Achieving a critical mass through either a gun-type or implosion weapon design can cause an explosive nuclear chain reaction within a fraction of a second to release enormous energy equivalent to thousands of tons of TNT.
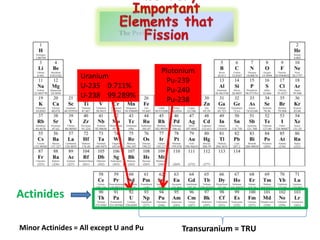
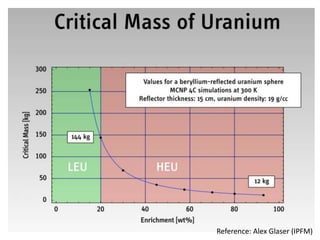
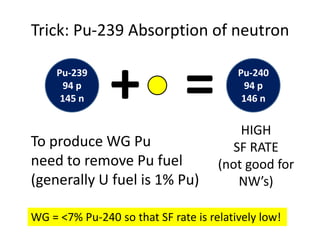
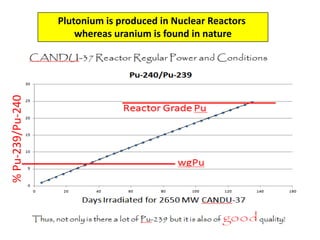
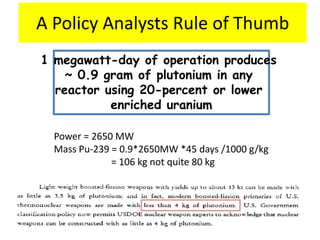
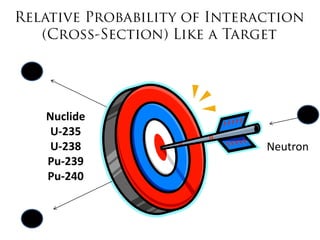
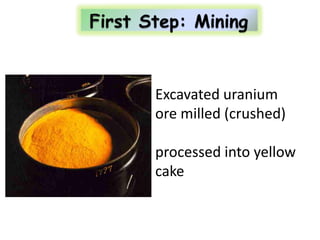
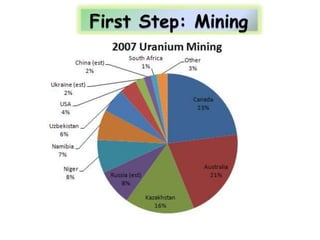
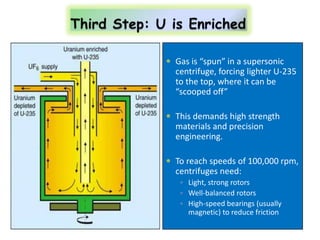
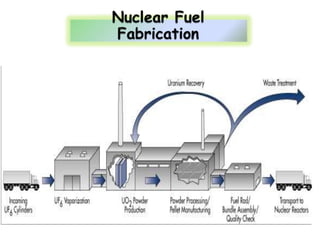
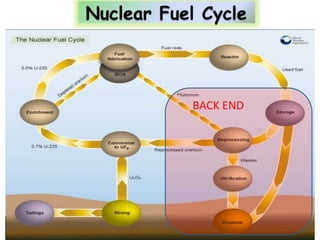
3. Enriching uranium from its natural 0.711% concentration of U-235 to weapons-grade highly enriched uranium above 90% U-235 is an extensive technical process that removes the U-238 isotope.

































































![1.E+07
1.E+06
PYRO Product
1.E+05
NOT IN PYRO
1.E+04 Pu-Odd
Pu-Even
1.E+03
Ci/MTHM
Np-237
Cs-137 + Sr-90
1.E+02
Fission Products Excluding
1.E+01 Tc-99 + I-129
TOTAL
1.E+00 TOTAL LOW
1.E-02 1.E-01 1.E+00 1.E+01 1.E+02 1.E+03 1.E+04 1.E+05 1.E+06 Am
1.E-01
1.E-02
1.E-03
Years After Discharge
: The radioactivity profile of SNF throughout time calculated by the author with ORNL’s Scale6 code system [SCALE6] for a Westinghouse 50 MWd/kg
HM and 4.5% enriched PWR fuel assembly. The dotted line indicates the time when the SNF cooled in reactor cooling ponds are moved to interim
storages such as dry casks. The actinides are generally represented by the thicker lines and thinner lines correspond to the actinides. Notice that
after the fission products (especially Cs-137 and Sr-90) decay the actinides, and Tc-99 and I-129 will start to dominate the profile. Notice also that
the pyroprocessing products will after several decades be at the level of hundreds of Ci and will be below the level that the IAEA considers self-
protective [Kang and von Hippel] since the fission products that would produce a self-protective dose are removed in pyroprocessing (see NOT IN
PYRO label).](https://image.slidesharecdn.com/vsf-module2-aug11-120828192237-phpapp02/85/Vsf-module-2-aug-11-126-320.jpg)