1) The document describes two experiments involving oxidation-reduction reactions. The first experiment measured the rate of decomposition of hydrogen peroxide over time. The second experiment measured the rate of a redox reaction between potassium iodide and persulfate ions.
2) Both experiments involved measuring the volume of gas produced over time to determine the amount of reactant consumed and the rate of reaction. Rate calculations were performed using the volume and time data.
3) Thermodynamic considerations and balanced chemical equations were provided to explain the redox reactions occurring in each experiment.
![:
2008 :
. q = 12°C ( H 2O2 )
2 H 2O2( aq) = 2 H 2O( l ) + O2( g ) :
( VM = 24 L.mol -1 )
. [ H 2O2 ]0 = 8, 0 ´10 -2 mol.L-1 VS = 500mL t =0
: (V )
O2
t ( min ) 0 4 8 12 16 20 24 28 32 36 40
VO2 ( mL ) 0 60 114 162 204 234 253 276 288 294 300
[ H 2O2 ] mol.L-1
. .1
. VO2 VM VS [ H 2O2 ]0 : t [ H 2O2 ] .2
. - .3
. [ H 2O2 ] = f ( t ) -
. -
. . t2 = 24 min t1 = 16 min -
. t1 2 -
. . [ H 2O2 ] q ' = 35°C .4
. :
2 H 2O2 (aq ) ® O2 ( g ) + 2 H 2O(l ) . .1
:
( mol )
. n = [ H 2O2 ]0 .VS = 8, 0 ´10-2 ´ 0,5
0 n 0 0
. n = 4 ´ 10-2 mol
x (t ) n - 2x (t ) x (t ) 2x ( t )
[ H 2O2 ] .2
xf n - 2x f xf 2x f
. VO2 VM VS [ H 2O2 ]0 : t
ni ( H 2O2 ) - 2 x ( t ) x (t )
[ H 2O2 ] = = [ H 2O2 ]0 - 2. n( H 2O2 ) ( t ) = ni ( H 2O2 ) - 2 x ( t ) : t
VS VS
2 VO2
. [ H 2O2 ] = [ H 2O2 ]0 - .VO : n ( O2 ) = =x :
VS .VM 2 VM
. - .3
1 2
. [ H 2O2 ] = 8,0 ´ 10-2 - .VO2 : [ H 2O2 ] = 8, 0 ´10-2 - .VO :
6 0, 5 ´ 24 2
1](https://image.slidesharecdn.com/unit01bac-121010055536-phpapp01/85/Unit01-bac-1-320.jpg)
![t ( min ) 0 4 8 12 16 20 24 28 32 36 40
[ H 2O2 ] ´10-2 mol.L-1 8,0 7,0 6,1 5,3 4,6 4,1 3,8 3,4 3,2 3,1 3,0
. [ H 2O2 ] = f ( t ) -
. -
1 dx
vvol = . :
VS dt
d [ H 2O2 ] 1 d 2 dx
. = ( n - 2x) = - :
dt VS dt VS dt
1 d [ H 2O2 ]
vvol = - . :
2 dt
d [ H 2O2 ]
.t [ H 2O2 ] = f ( t ) :
dt
. t2 = 24 min t1 = 16 min -
1 ( 7, 0 - 4, 6 ) ´ 10
-3
vvol ( t1 ) = - . = 7, 5 ´10 -4 mol.L-1 .min -1
2 16 - 0
1 ( 7, 0 - 5,5 ) ´ 10
-3
vvol ( t2 ) = - . = 3,1´ 10-5 mol.L-1.min -1
2 24 - 0
.
. t1 2 -
[ H 2O2 ]0 xmax
( ) . [ H 2O2 ] ( t1/2 ) = : x ( t1/ 2 ) = :
2 2
0, 08
.( ) t1/ 2 » 20 min : [ H 2O2 ] ( t1/2 ) = = 40mmol .L-1 :
2
. ( ) q ' = 35°C [ H 2O2 ] .4
,
2008 :
.( )
30mL m = 36mg Mg ( S )
.
. .1
(H (
+
aq ) )
/ H 2( g ) : .2
(
. Mg 2 + ( aq ) / Mg( s ) )
: .3
t ( min ) 0 2 4 6 8 10 12 14 16 18
VH 2 ( mL ) 0 12,0 19,2 25,2 28,8 32,4 34,8 36,0 37,2 37,2
x ( mol )
2](https://image.slidesharecdn.com/unit01bac-121010055536-phpapp01/85/Unit01-bac-2-320.jpg)

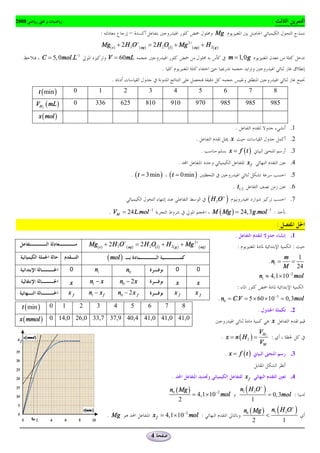
![. n2 = 50mmol : n2 = C2 .V2 = 1, 0 ´ 50 ´10-3 = 5 ´10-2 mol : I-
. .3
. xmax = n1 = 10mmol n2 - 2 xmax = 0 n1 - xmax = 0 :
. S 2O 8-2 :
. t1 .4
2
n1 10 xf
nS O -2 = = = 5mmol : x ( t1/ 2 ) = :
2 8
2 2 2
. t1/2 = 17,5 min : nS O-2 = 5mmol
2 8
. t1 .5
2
t1
2
2n + n x1/ 2 2 x1/ 2 n - 2x1/2 n -x
éK + ù = 1 2
ë û V +V [I2 ] = é SO4-2 ù = éI - ù = 2
ë û V +V é S2O8-2 ù = 1 1/2
ë û V +V
V1 + V2 ë û V +V
1 2 1 2 1 2 1 2
20 + 50 5 10 50 - 10 10 - 5
= = = = =
100 100 100 100 100
é K ù = 0, 7 mol.L-1
ë û
+
[ I 2 ] = 0, 05mol.L-1 é SO4 ù = 0,1mol.L-1
ë
-2
û é I - ù = 0, 4mol.L-1
ë û é S2O8-2 ù = 0,05mol.L-1
ë û
. t = 10 min .6
dx dnS O-2
. =- 2 8
: nS O-2 = n1 - x :
dt dt 2 8
1 dx 1 dnS2O8-2
vvol = =- :
V dt V dt
dnS O-2
. t = 10 min 2 8
dt
1 6,6 - 9,3
vvol = - = 2,7mmol.L-1.min -1 :
0,1 10 - 0
2009 :
: (I )
-
(S O )
2
2-
8
S 2O82- ( aq) + 2 I -( aq ) = 2SO42- ( aq ) + I 2( aq)
V1 = 100mL (t = 0) (q = 35°C ) .I
V2 = 100mL C1 = 4, 0 ´ 10-2 mol.L-1 ( 2K +
+ S 2O82 - )
. VT = 200mL C2 = 8, 0 ´ 10-2 mol.L-1 (K +
+ I-)
. -
( I2 ) [ I2 ] V2 V1 C1 é S2O82- ù
ë û -
.
(I )
-
(S O )
2
2-
8 (t = 0) é S 2O82- ù
ë û0 -
V0 = 10mL . ti ........ t2 t1 .II
( 2Na +
+ S2 O32 - ) ti
6](https://image.slidesharecdn.com/unit01bac-121010055536-phpapp01/85/Unit01-bac-6-320.jpg)
![: V' C ' = 1,5 ´ 10-2 mol.L-1
t ( min ) 0 5 10 15 20 30 45 60
V ' ( ml ) 0 4,0 6,7 8,7 10,4 13,1 15,3 16,7
[ I 2 ] ( mmol / l )
-
. I 2( aq) / I -( aq) S 4O62-( aq) / S 2O32-( aq ) : -
. ·
1 C '´ V '
[ I2 ] = ´ : -
2 V0
. -
. [ I2 ] = f (t ) -
. ( t = 20 min ) -
. :
. - .I
2- - 2-
S 2O 8 ( aq ) + 2I ( aq ) = 2SO 4 ( aq ) + I 2( aq) S 2O 2-
8
( mmol ) . n1 = C1.V1 = 4, 0 ´10-2 ´10-1 :
n1 = 4, 0 ´ 10 -3 mol = 4mmol
0 4 8 0 0
: I-
x (t ) 4 - x (t ) 8 - 2x (t ) 2x ( t ) x (t )
. n2 = C2 .V2 = 8, 0 ´10-2 ´10-1
xf 4-xf 8 - 2x f 2x f xf
n2 = 8, 0 ´ 10-3 mol = 8mmol
. é S2O82- ù
ë û -
n - x C1.V1 x
é S 2O82- ù = 1
ë û = - : ( )
VT V1 + V2 V1 + V2
C .V
é S 2O82- ù = 1 1 - [ I 2 ] :
x
ë û V +V . [ I2 ] = : nI 2 = x :
1 2 V1 + V2
. é S 2O82- ù
ë û0 -
4 ´10-2 ´10-1 C .V
. é S2O82- ù =
ë û0 -1 -1
= 2 ´10-2 mol.L-1 : é S 2O82- ù = 1 1 :
ë û0 V + V
10 + 10 1 2
. ( I2 ) - .II
. -
2- 2- -
. 2 S 2O 3 ( aq ) ® S4 O 6 ( aq ) + 2e :
. I 2(aq ) + 2e - ® 2 I - (aq ) :
2S 2O32-( aq ) + I 2( aq) ® S4O6 - ( aq ) + 2 I - ( aq ) :
2
. -
2S 2O32-( aq ) + I 2( aq) ® S4O6 - ( aq ) + 2 I - ( aq )
2
.
( mol ) n '- 2 xE = 0 n0 - x E = 0 :
0 n' n0 0 0 n'
xE = n0 = :
xE n '- 2 xE n0 - x E xE 2x E 2
7](https://image.slidesharecdn.com/unit01bac-121010055536-phpapp01/85/Unit01-bac-7-320.jpg)
![t ( min ) 1 C '.V ' n0
0 5 10 15 20 30 45 60 . [ I2 ] = . : [ I2 ] = :
2 V0
[ I 2 ] ( mmol.L-1 )
V0
0 3,0 5,0 6,5 7,8 9,8 11,5 12,5
. -
1,5 ´10-2 1 C '.V '
( mmol.L-1 [ I2 ] mL V' ) . [ I2 ] = V ' = 0, 75 ´ V ' : [ I2 ] = . :
2 ´10 ´10-3 2 V0
. [ I2 ] = f (t ) -
.
. ( t = 20 min ) -
d æ x ö d [I2 ] 1 dx
. vvol = ç ÷= : vvol = :
dt è VT ø dt VT dt
d [ I2 ]
. t = 20 min vvol =
dt
7,8 - 3, 2
. v vol = = 0, 23mmol .min -1 .L-1 :
20 - 0
2009 :
2,0g . (H +
+ Cl - )
. C = 1,0 ´10-1 mol.L-1 100mL CaCO3
:
t (s) 20 60 100
(1L )
P(CO2 ) ( Pa ) 2280 5560 7170
: T = 25°C
n( CO2 ) ( mol ) :
x ( mol ) . CaCO3( s ) + 2 H + ( aq ) = CO2( g ) + H 2O( l ) + Ca 2+ ( aq )
.1
. ( x) (n )
CO2 .2
. ( P.V = n.R.T ) .3
. 1L = 10-3 m3 R = 8,32SI . x = f (t ) .4
:
: (H )+
t (s) 20 60 100 . (n H + )
( )
.1
é H + ù ( mol.L-1 )
ë û
0,080 0,056 0,040 (n H + ) .2
( )
n H + ( mol ) . ( n0 ) ( x)
( )
x ( mol )
. ( x) .3
x = f (t ) .4
. .5
. t1/ 2 .6
. t = 50s .7
. M ( Ca ) = 40 g .mol -1 M ( C ) = 12 g .mol -1 M ( O ) = 16 g .mol -1
8](https://image.slidesharecdn.com/unit01bac-121010055536-phpapp01/85/Unit01-bac-8-320.jpg)
![2009 :
. ( H 2O2( aq) )
10L (1L ) (10V )
. Vm = 22, 4 L.mol -1
. 2 H 2O2( aq) = 2 H 2O( l ) + O2( g ) : .1
. C = 0,893mol.L-1 : -
. 100mL V1 -
·
. C1 = 0,1mol.L-1 V1 ·
(K (
+
aq )
+ MnO4-( aq ) ) 20mL (10V ) .2
. VE = 38mL C0 = 0, 02mol.L-1
(O ( ) / H O ( ) ) :
2 g 2 2 aq -
(
. MnO4 ( aq ) / Mn 2 + ( aq )
-
)
. -
. :
. C = 0,893mol.L-1 : - .1
VO2 10
. nO2 = = » 0, 4464mol : (1L )
Vm 22, 4
ni ( H 2O2 ) = 2n f ( O2 ) = 2 ´ 0, 4464 » 0,893mol :
ni ( H 2O2 ) 0,893
.C= = = 0,893mol.L-1 :
VH 2O2 1
. -
. V1 -
0,1 C1
. V1 = .100 = 11, 2mL : V1 = .V : CV1 = C1V :
0,893 C
. - .2
( )
. 2 ´ MnO4 ( aq ) + 8H + ( aq ) + 5e - ® Mn 2 + ( aq ) + 4 H 2O( l ) :
-
. 5 ´ ( H 2O2( aq ) ® O2( g ) + 2 H + ( aq ) + 2e - ) :
. 2MnO4 ( aq ) + 5H 2O2( aq ) + 6 H +( aq) ® 2Mn 2+( aq) + 5O2( g ) + 8H 2O( l ) :
-
. -
n ( MnO4- ) n ( H 2 O2 )
. xmax = = : n ( H 2O2 ) - 5 xmax = 0 n ( MnO4- ) - 2 xmax = 0 :
2 5
5 5
. n ( H 2 O2 )( t ) = C0 .VE : V0 = 20mL n ( H 2 O2 ) n ( H 2 O2 ) = n ( MnO4- )
2 2
5 C .V 5 0, 02 ´ 38
C ' = [ H 2 O2 ] = . 0 E = . = 0, 095mol.L-1 :
2 V0 2 20
10](https://image.slidesharecdn.com/unit01bac-121010055536-phpapp01/85/Unit01-bac-10-320.jpg)



![. I2 -
1 dnI 2 d [ I 2 ]
. vvol ( I 2 ) = = : I2
V dt dt
.t [ I2 ]
. I2 -
. I2 [ I2 ]
) : .3
.(
: .4
.5
.
:
·
·
2010 :
( 2K (
+
aq )
+ S2 O8-2 ( aq ) ) V1 = 200mL t =0
C2 = 4, 0 ´ 10-1 mol.L-1 (K ( +
aq )
+ I -( aq ) ) V2 = 200mL C1 = 4, 00 ´ 10-2 mol.L-1
(
. I 2( aq ) / I - ( aq )) (S O 2
-2
8 ( aq )
-
/ SO4 2( aq ) :) ( Ox / Re d ) .1
. -
. . -
. I2 .2
. (1- ) [ I2 ] = f (t )
-
. t = t1 -
2
( 1- ) .3
V = 10mL
( )
( 2 Na ( +
aq )
/ S 2O3-2( aq ) )
. C ' = 1, 0 ´ 10-2 mol.L-1
. I 2( aq) + 2S 2O32-( aq ) = 2 I - ( aq) + 2 S4O62-( aq) :
. -
.E [ I2 ] : . C ' VE V : [ I2 ] -
. t = 1, 2 min VE -
. :
. - .1
. S 2O 8-2(aq ) + 2e - = 2SO 4-2( aq ) : 2I - (aq ) = I 2(aq ) + 2e - :
14](https://image.slidesharecdn.com/unit01bac-121010055536-phpapp01/85/Unit01-bac-14-320.jpg)
![. S 2O 8-2(aq ) + 2I - (aq ) = 2SO 4-2( aq ) + I 2( aq ) :
: -
S 2O 8-2(aq ) + 2I - (aq ) = 2SO 4-2( aq ) + I 2( aq ) :
mmol n1 = C 1 V 1 = 4 ´ 10-2 ´ 0, 2 = 8 ´ 10-3 mol
.
0 8 80 0 0 . n1 = 8mmol :
x 8-x 80 - 2x 2x x :
xf 8- xf 80 - 2x f 2x f xf n 2 = C 2 V 2 = 4 ´ 10-1 ´ 0, 2 = 8 ´ 10-2 mol
.
n 2 = 80mmol :
. S 2O 8-2 : x max = 8 80 - 2 xmax = 0 8 - x max = 0 :
. - .2
[ I 2 ]f 20
. t1/ 2 = 0,84 min : [ I 2 ]1/ 2 = = = 10mmol .L-1
2 2
. t1/2 -
10 - 3 1 dx d [ I 2 ]
v vol = » 8,3mmolL .L-1 min -1 : . t1/2 v vol = . = :
0,84 - 0 V total dt dt
. : - .3
. C ' VE V [I 2 ] -
nI2 VE 1 nI 2 nS O -2
. [I 2 ] = = .C ' : n I 2 = C '. E :
V = 2 3
:
V 2V 2 1 2
. t = 1, 2 min VE -
2 [ I 2 ]V VE
VE = : [I 2 ] = .C ' : [ I 2 ] = 13mmol .L-1 : t = 1, 2 min
C' 2V
2 ´13 ´10 -3 ´10
. V E = 26mL : VE = :
10-2
2010 :
V2 = 100mL C1 = 4, 5 ´ 10-2 mol.L-1 H 2 O2 V1 = 100mL (S)
(
. H 2O2 ( aq ) / H 2O(l ) ) (I ( 2 aq ) )
/ I - ( aq ) : . C2 = 2, 0 ´ 10-1 mol.L-1 (K ( +
aq )
+ I -( aq ) )
. - .1
. -
t = 3min V = 20mL (S) .2
C = 1, 0mol.L-1 ( 2 Na (
+
aq )
/ S2O32 -( aq ) ) I 2( aq)
. VE
: .3
2- - 2-
. I 2( aq) + 2S 2O 3 ( aq ) = 2I ( aq ) + S4 O 6 ( aq )
CVE
. [I2 ] = : t
2V
(1- ) .4
. [ I2 ] f -
. t = 8 min I2 -
. t = 8 min -
1-
15](https://image.slidesharecdn.com/unit01bac-121010055536-phpapp01/85/Unit01-bac-15-320.jpg)
![. :
. - .1
+ -
. 2 I - ( aq ) ® I 2 ( aq ) + 2e - : . H 2O2( aq) + 2 H ( aq ) + 2e ® 2 H 2O( l ) :
. H 2O2 ( aq ) + 2 I - ( aq ) + 2 H + ( aq ) ® 2 H 2O( l ) + I 2 ( aq ) :
: -
:
H 2O2( aq) + 2 I - ( aq ) + 2 H +( aq) ® I 2( aq ) + 2 H 2O( l )
n1 = C1 .V1 = 4,5 ´ 10 -2 ´ 0,1
( mmol ) . n1 = 4,5 ´ 10-3 mol :
0 n1 = 4,5 n2 = 20 0 :
x n1 - x n2 - 2 x x n2 = C2 ´ V2 = 2, 0 ´ 10-1 ´ 0,1
xf n1 - x f n2 - 2 x f xf . n2 = 2, 0 ´ 10-2 mol :
·
-2 -2 -3 -3
. xmax = 10 mol : 2, 0 ´ 10 - 2 xmax = 0 xmax = 4,5 ´ 10 mol : 4,5 ´ 10 - xmax = 0
. H 2 O2 xmax = 4,5 ´ 10-3 mol :
.( )t I2 .2
CVE
. [I2 ] = : .3
2V
n ( I 2 ) n ( S2 O3 )
-2
[ I 2 ] ( mmol.L )
1
-1
[ I 2 ].V = CVE : = :
2 1 2
CVE
[ I2 ] f [ I2 ] f = 5, 6 ´ 4 = 22, 4mmol .L-1 . [ I 2 ] =
2V
:
. [ I2 ] f - .4
. [ I 2 ] f = 5, 6 ´ 4 = 22, 4mmol .L-1 :
. t = 8 min I2 -
d [ I2 ]
. t = 8 min vvol ( I 2 ) = :
dt
18 - 11, 6
vvol ( I 2 ) = = 0,8mmol.L-1.min -1 :
8-0
. t = 8 min -
dnH2O2 dx
. vH 2O2 = 0, 2 ´ 0,8 = 0,16mmol.L-1.min -1 : vH 2O2 = - = = V .vvol = V .vvol ( I 2 ) :
dt dt
2011 :
.
. 2 H 2O2( aq) = 2 H 2O( l) + O2( g ) :
. .1
:
10L 1L ) 10V S0 500mL -
( VM = 22, 4 L / mol
: -
16](https://image.slidesharecdn.com/unit01bac-121010055536-phpapp01/85/Unit01-bac-16-320.jpg)


