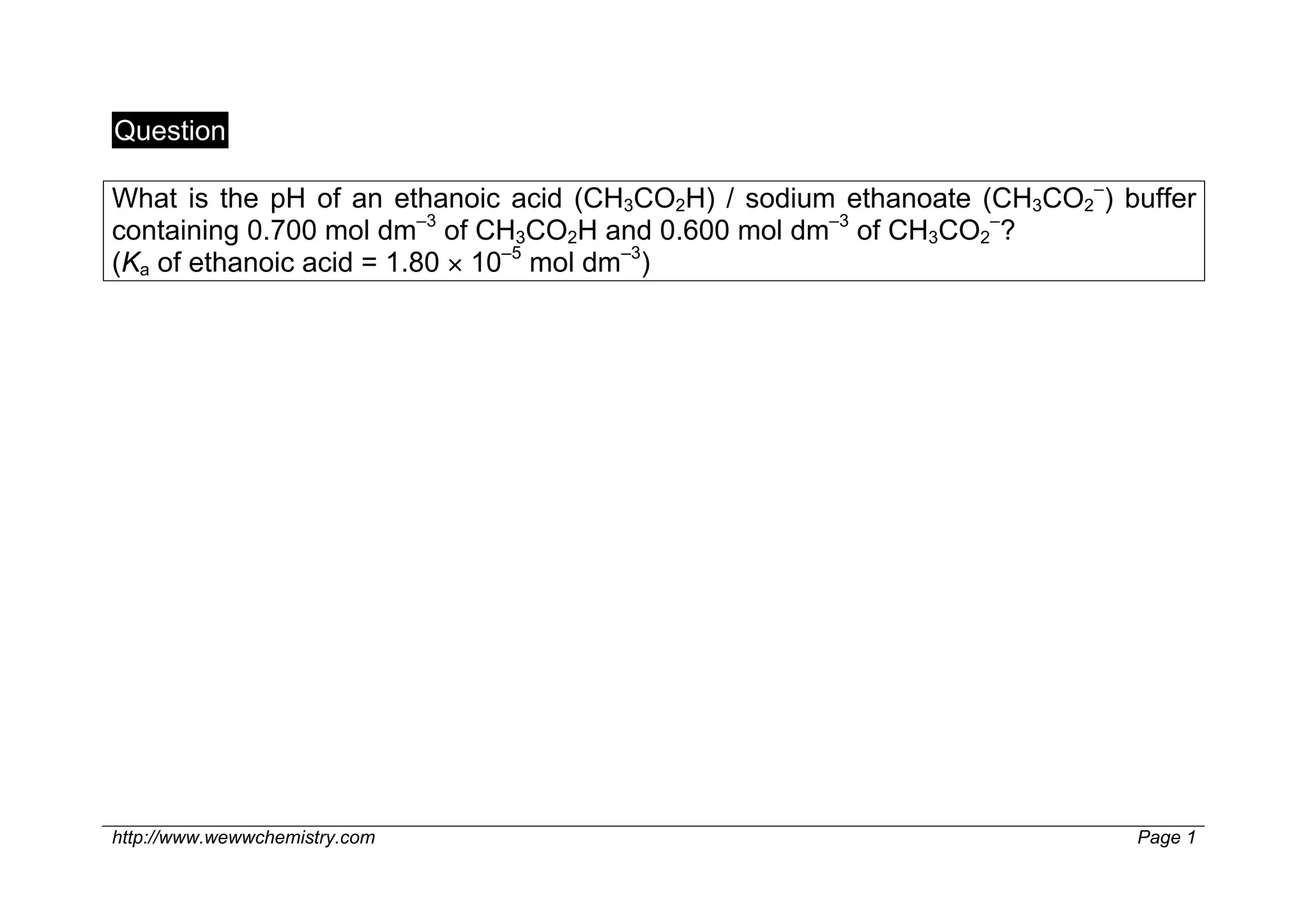
1) The pH of an ethanoic acid/sodium ethanoate buffer containing 0.700 mol dm–3 of CH3CO2H and 0.600 mol dm–3 of CH3CO2– is 4.68.
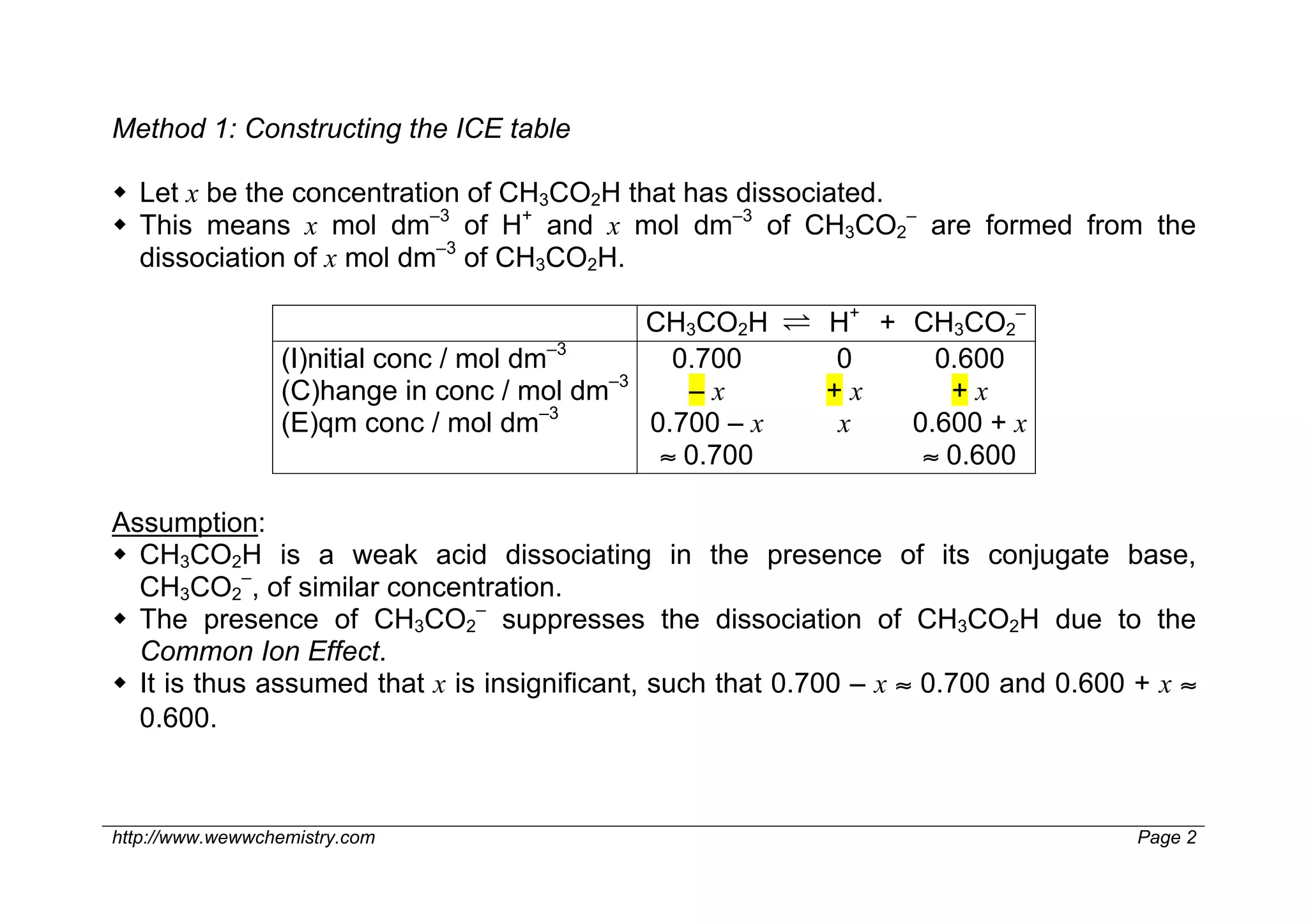
2) This is calculated by constructing an ICE table to determine the equilibrium concentration of H+ ions, then taking the negative log of the Ka expression.
3) Alternatively, the pH can be determined directly by substituting the initial concentrations of CH3CO2H and CH3CO2– into the Henderson-Hasselbalch equation, since x is assumed to be insignificant.
![[CH3CO2–][ H+]
Ka =
[CH3CO2H]
(0.600 + x)(x)
1.80 × 10–5 =
(0.700 – x)
0.600 x
1.80 × 10–5 ≈
0.700
x = 2.10 × 10–5
∴ pH of buffer = –lg(2.10 × 10–5) = 4.68
http://www.wewwchemistry.com Page 3](https://image.slidesharecdn.com/2012-12-22tocalculatebufferph-121223111359-phpapp02/75/To-Calculate-the-pH-of-a-Buffer-3-2048.jpg)
![Method 2:
Substituting given data into the Henderson-Hasselbalch equation (H Eqn) directly
[CH3CO2–]
pH = pKa + lg
[CH3CO2H]
The H Eqn is derived from the Ka expression.
Like the Ka expression, all concentration terms in the H Eqn are equilibrium
concentrations.
If we substitute the equilibrium concentrations from the ICE table in Method 1 into the
H Eqn, we have the expression shaded in yellow (page 5). Normally, this step is
skipped, and many students simply substitute the initial concentrations of CH3CO2H
and CH3CO2– given in the question without fully understanding why.
Note that initial concentrations of CH3CO2H and CH3CO2– are substituted directly into
the H Eqn as x is assumed to be insignificant compared to the initial concentrations
of CH3CO2H and CH3CO2–.
http://www.wewwchemistry.com Page 4](https://image.slidesharecdn.com/2012-12-22tocalculatebufferph-121223111359-phpapp02/75/To-Calculate-the-pH-of-a-Buffer-4-2048.jpg)
![[CH3CO2–]
pH = pKa + lg
[CH3CO2H]
–5 (0.600 + x)
pH = –lg(1.80 × 10 ) + lg
(0.700 – x)
–5 0.600
pH ≈ –lg(1.80 × 10 ) + lg
0.700
= 4.68
http://www.wewwchemistry.com Page 5](https://image.slidesharecdn.com/2012-12-22tocalculatebufferph-121223111359-phpapp02/75/To-Calculate-the-pH-of-a-Buffer-5-2048.jpg)