More Related Content
PPTX
TLC Overview (1).pptx thin layer chromatography PPTX
Thin layer chromatography PPTX
Thin layer chromatography(tlc) PPTX
Thin-Layer-Chromatography-TLC---pptx...... PPT
Introduction to Chromatography and HPLC PPTX
3. Thin Layer CHROMATOGRAPHY Bio Techniques.pptx PPTX
Thin layer chromatography.(Abhni Gupta)pptx PDF
Thin_layer_chromatography zoology pg student Similar to TLC_Presentation thin layer chromatography
PPTX
Chromatography techniques PDF
Thin Layer Chromatography for Biotechnology & Botany Sem-2 Students PPTX
PPTX
Tlc(thin layer chromatography ) PPTX
THIN LAYER CHROMATOGRAPHY PPTX
PPT ON Thin layer chromatography ,Principle,System Components,Procedure,Analysis PDF
Thin Layer Chromatography (TLC) on Slideshare by Raj Kumar Mandal. PPTX
hhhjwjwjwjejejejebejsebjebwjejeienwiejwjwj.pptx PPTX
PPT
THIN LAYER CHROMATOGRAPHY.ppt PPT
Chromatography techniquesssssssssssss.ppt PPSX
T.Y.B.Sc. Thin layer chromatography PPT.ppsx PPTX
THIN LjsjsjsnsjsjsjejsjwjsAYER CROMATOGRAPHY.pptx PPTX
THIN LAYER CHROMATOGRAPHY ppt.pptx PPTX
THIN LAYER CHROMATOGRAPHY.pptx PPTX
TLC_Presentation 2.pptx thin layer chromatography PPTX
Introduction to Pharmaceutical analysis.pptx PPTX
Introduction to Pharmaceutical analysis.pptx PPTX
Thin layer chromatography - bio instrumentation PPTX
LECTURE5 TLC.pptx thin layer chromatography Recently uploaded
PDF
NAVIGATE PHARMACY CAREER OPPORTUNITIES.pdf PDF
Projecte de la porta de primer B: L'antic Egipte PDF
DHA/HAAD/MOH/DOH OPTOMETRY MCQ PYQ. .pdf PDF
Analyzing the data of your initial survey PPTX
Filipino 11-Tekstong Prosidyural-Final.pptx PPTX
Semester 6 unit 2 Atopic dermatitis.pptx PPTX
Pig- piggy bank in Big Data Analytics.ppt.pptx PDF
M.Sc. Nonchordates Complete Syllabus PPT | All Important Topics Covered PPTX
How to Configure Push & Pull Rule in Odoo 18 Inventory PDF
Projecte de la porta de la classe de primer A: Mar i cel. PPTX
AI_in_Daily_Life_Presentation and more.pptx PPTX
PURPOSIVE SAMPLING IN EDUCATIONAL RESEARCH RACHITHRA RK.pptx PPTX
Kochi MuleSoftMeetup_UnlockingMCPServer.pptx PDF
The Tale of Melon City poem ppt by Sahasra PDF
Blood Group Incompatibility: Rh Factor and Erythroblastosis Fetalis PPTX
Campfens "The Data Qualify Challenge: Publishers, institutions, and funders r... PPTX
Limpitlaw "Licensing: From Mindset to Milestones" PDF
Models of Teaching - TNTEU - B.Ed I Semester - Teaching and Learning - BD1TL ... PPTX
ICH Harmonization A Global Pathway to Unified Drug Regulation.pptx PDF
Projecte de la porta d'i5B: Els animals marins TLC_Presentation thin layer chromatography
- 1.
- 2.
- 3.
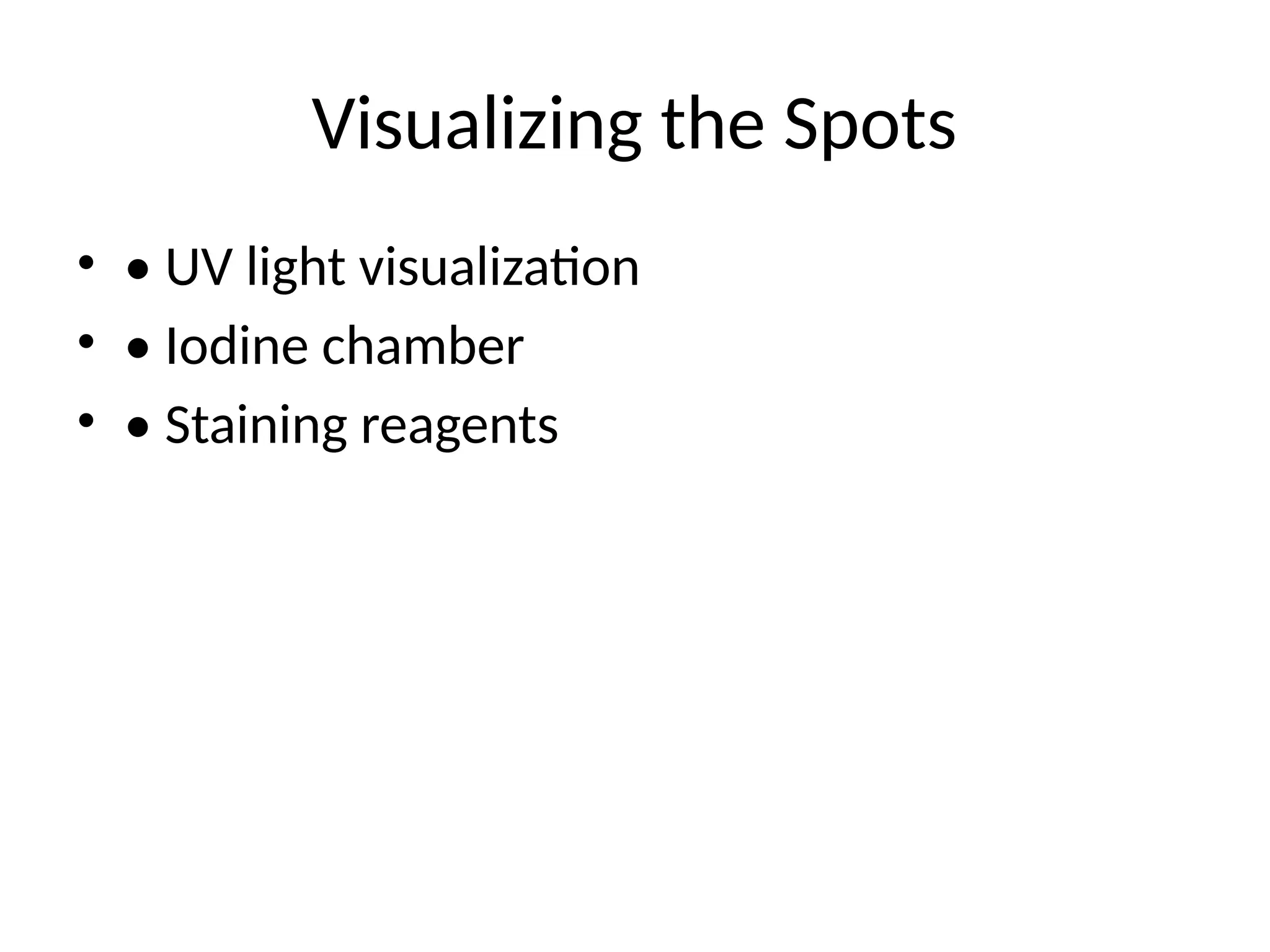
What is TLC?
•• Definition of Thin Layer Chromatography
• • Diagram of a TLC plate (insert image if
needed)
- 4.
- 5.
Components of TLC
•• Stationary phase: Silica gel or alumina
• • Mobile phase: Solvent
• • Sample: Compound to be separated
- 6.
- 7.
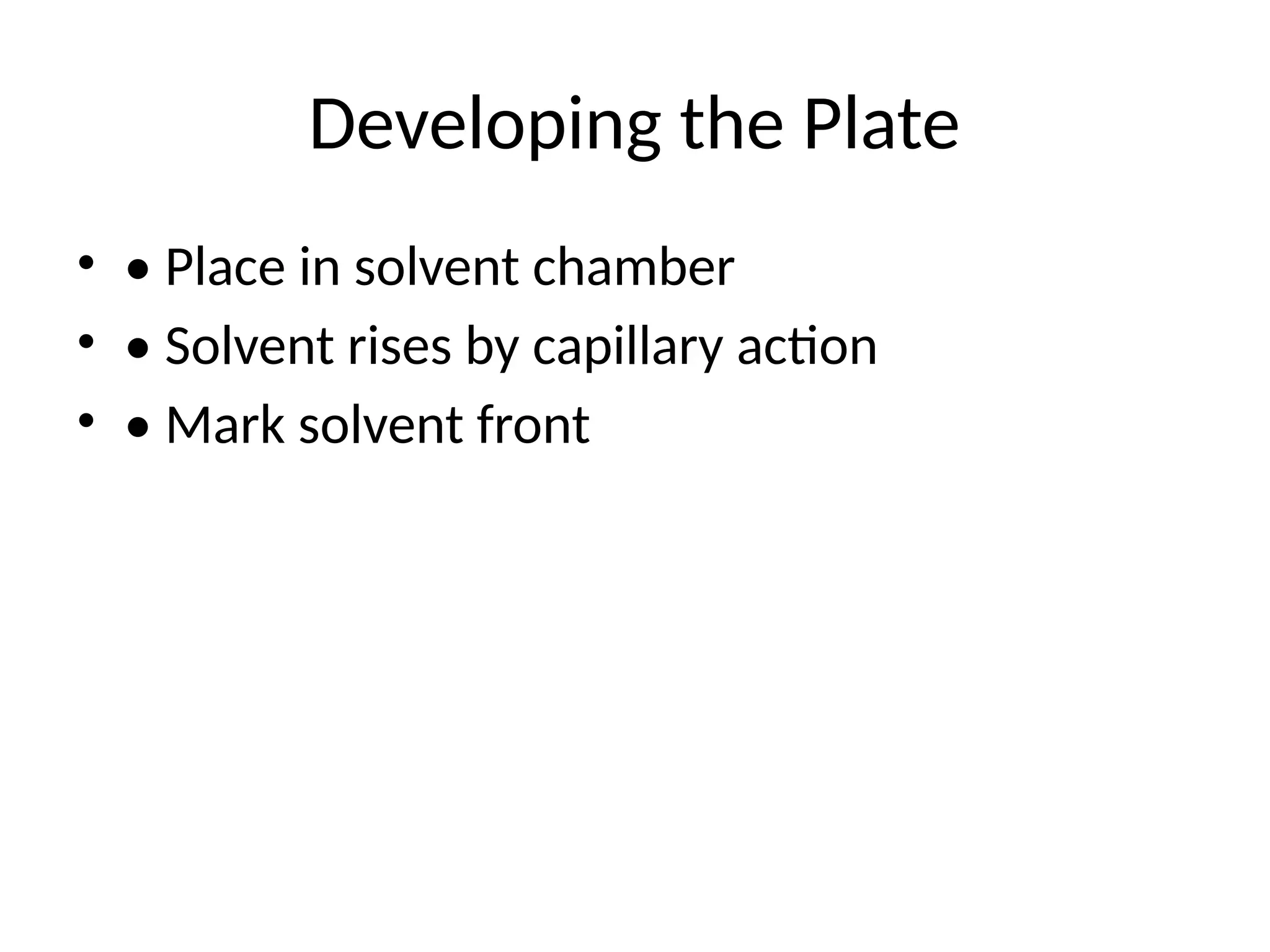
Preparing the TLCPlate
• • Coating and drying
• • Activation by heating
• • Marking baseline
- 8.
- 9.
- 10.
- 11.
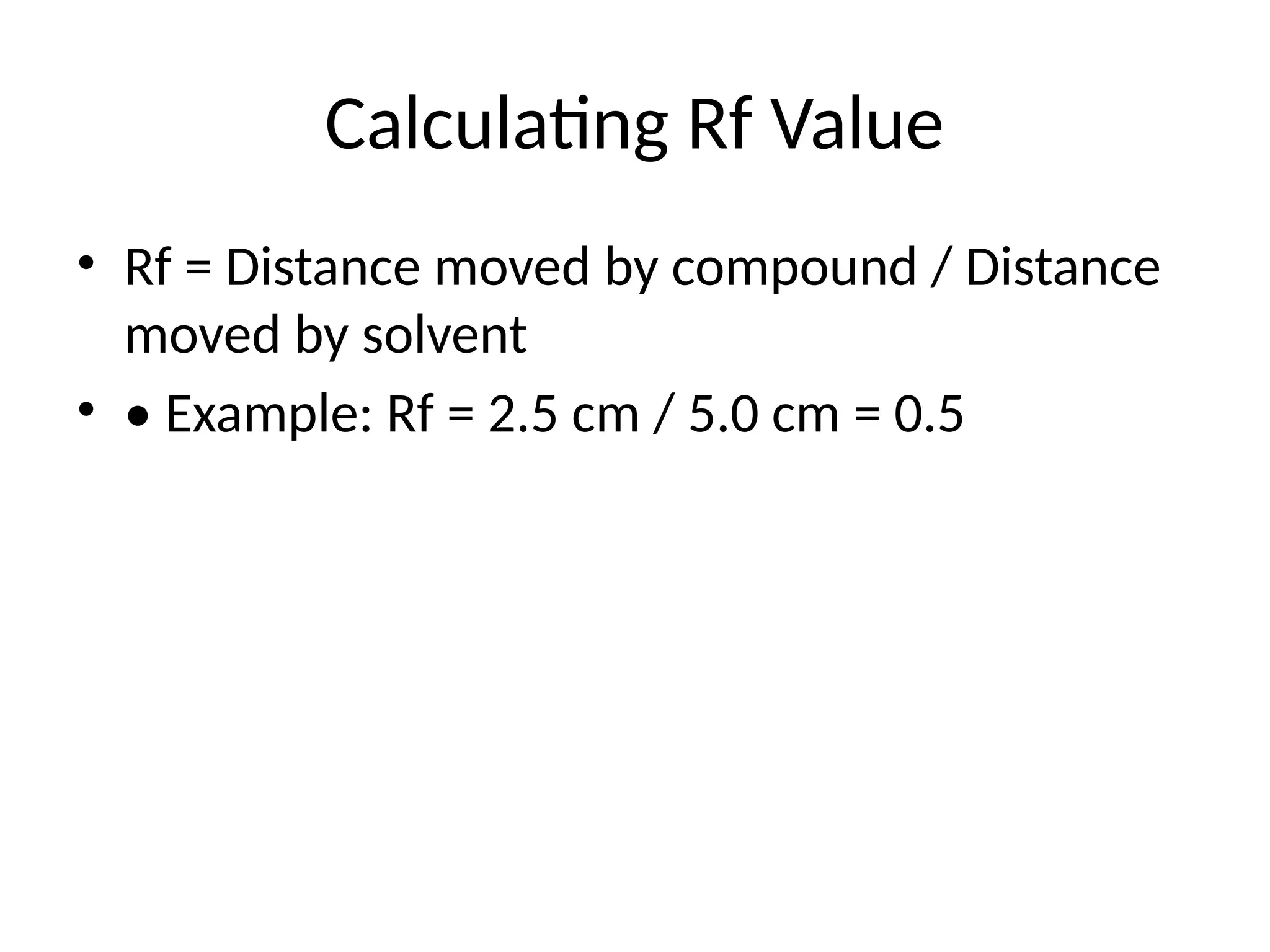
Calculating Rf Value
•Rf = Distance moved by compound / Distance
moved by solvent
• • Example: Rf = 2.5 cm / 5.0 cm = 0.5
- 12.
- 13.
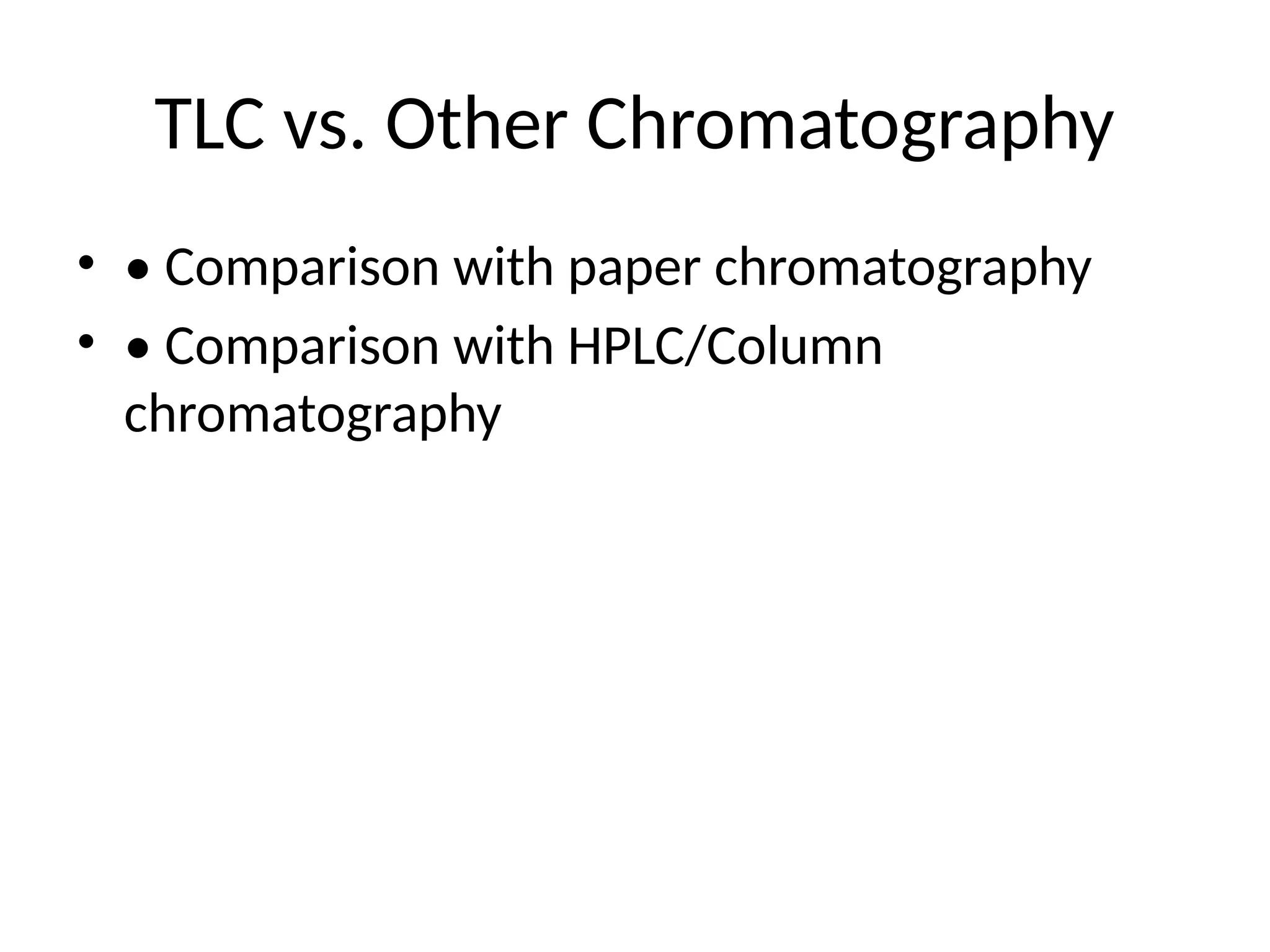
TLC vs. OtherChromatography
• • Comparison with paper chromatography
• • Comparison with HPLC/Column
chromatography
- 14.
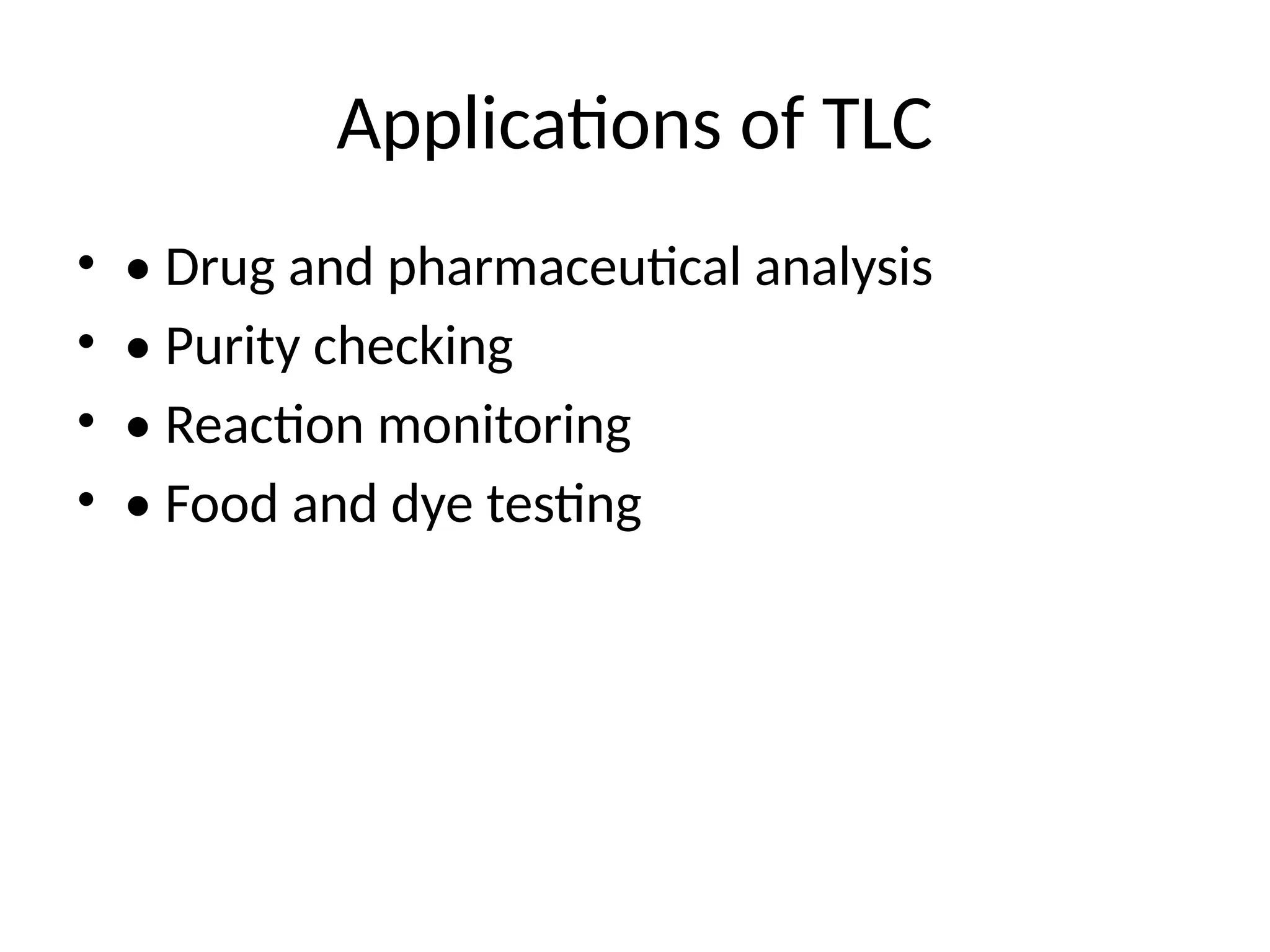
Applications of TLC
•• Drug and pharmaceutical analysis
• • Purity checking
• • Reaction monitoring
• • Food and dye testing
- 15.
- 16.
- 17.
TLC in ModernResearch
• • TLC-MS coupling
• • Preparative TLC
• • Forensic applications
- 18.
- 19.
Summary
• • TLCseparates mixtures based on polarity
• • Quick and cost-effective
• • Useful in various fields
- 20.