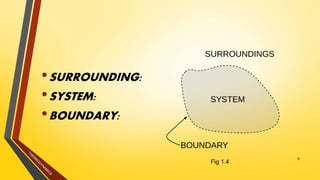
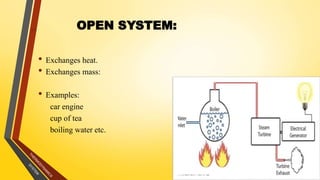
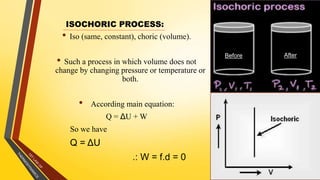
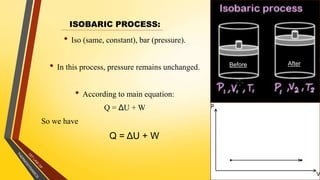
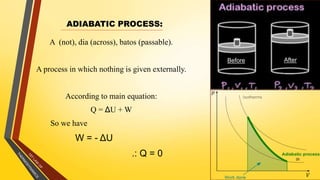
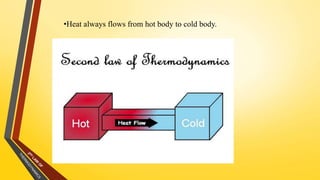
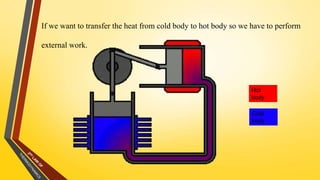
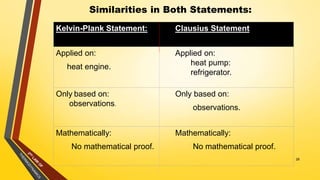
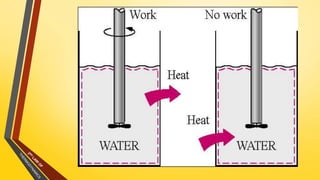
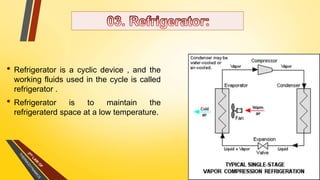
The document discusses the first and second laws of thermodynamics and provides examples of each. It defines the first law as stating that energy cannot be created or destroyed, only transferred or changed in form. Examples of applications of the first law include isothermal, isochoric, isobaric, and adiabatic processes. The second law is defined as heat not being able to spontaneously flow from a colder to a hotter body without external work. Both the Kelvin-Plank and Clausius statements of the second law are described as indicating it is impossible to have a cyclical heat engine or heat pump with 100% efficiency or one that transfers heat from a lower to higher temperature body without external work.