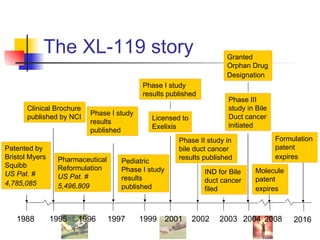
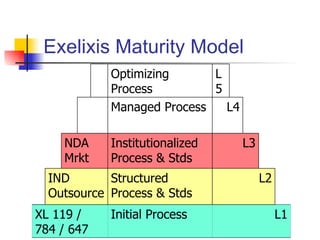
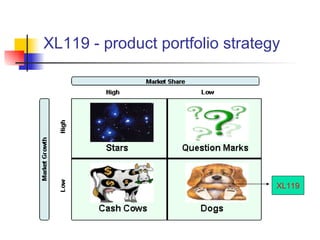
The document outlines the development timeline of XL-119, starting from its discovery by Bristol-Myers Squibb in 1986 and covering various clinical trials, licensing to Exelixis, and the drug's efficacy in bile duct cancer. It highlights challenges in formulation, funding, and operational risks while noting the drug's orphan status that provides market protection for seven years. The document also identifies potential market opportunities and competitive risks associated with the treatment of bile duct cancer.