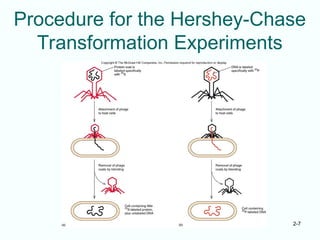
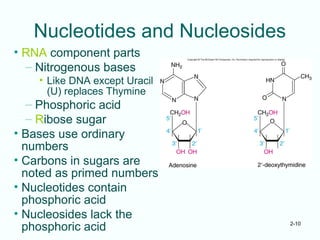
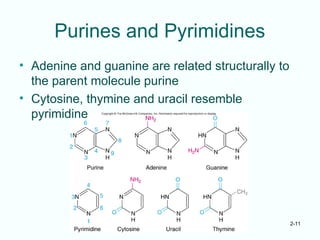
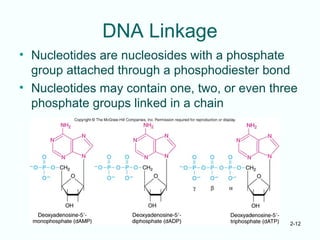
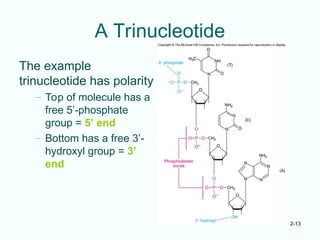
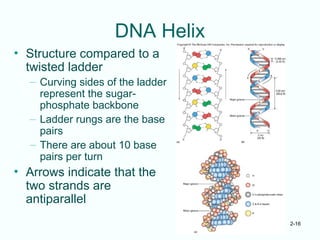
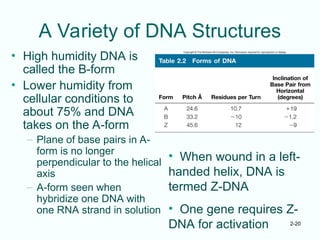
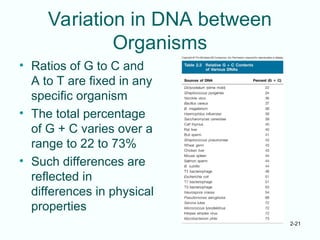
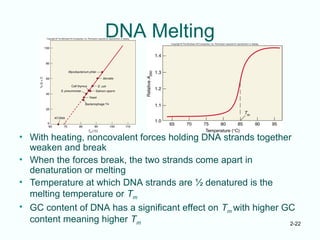
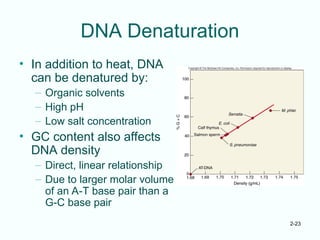
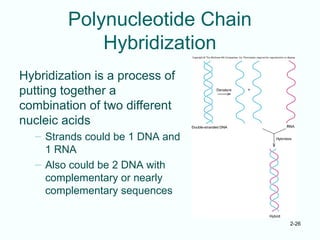
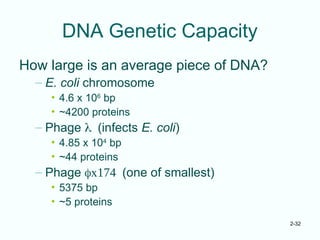
The document outlines the historical progression and understanding of genetic material, highlighting key experiments by figures like Miescher, Griffith, Watson, and Crick that established DNA as the genetic material. It discusses the structure of DNA, its components, hybridization, and differences in DNA between organisms, as well as concepts like the c-value paradox. Additionally, it explores the size and genetic capacity of DNA, indicating that complexity does not always correlate with gene number.