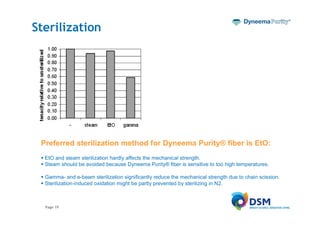
Dyneema Purity® fiber is a medical grade fiber made from ultra high molecular weight polyethylene. It is the strongest fiber available for medical use. The document discusses the properties of Dyneema Purity® fiber that make it suitable for applications such as sutures and ligament repair, including its high strength, flexibility, durability, and biocompatibility. Over 10 million patients have had medical devices implanted using Dyneema Purity® fiber due to its unique combination of properties.








![Chemical resistance
Chemical Conditions Tensile
strength
Conc. [%] Temp. Exp. time retention
[ºC] [hr]
Inorganic acids
HCl 10 20 5000 90-100 %
HNO3 10 20 5000
H2SO4 0.24 60 168
Ultra High Molecular Weight Polyethylene
Organic acids
CH3COOH 100 20 5000 90-100 %
95% crystalline
Bases No chemical groups
Ca(OH)2 0.25 60 168 90-100 %
Hydrophobic (Surface tension 27 mN/m)
NaOH 10 20 5000
Organic solvents
Very resistant against chemicals
Ethanol 100 20 5000 90-100 %
Acetone 100 20 5000
Trichloromethane 100 20 5000
Others
Distilled water 100 20 5000 90-100 %
Page 18](https://image.slidesharecdn.com/summarydyneemapurity2011-111116061455-phpapp01/85/Summary-dyneema-purity-2011-19-320.jpg)