1) Cohort studies follow groups of individuals over time to examine exposure-outcome relationships. Case-control studies compare exposures in individuals with an outcome to those without.
2) Cohort studies directly observe the temporal sequence of exposure and outcome. Case-control studies rely on recall of past exposures.
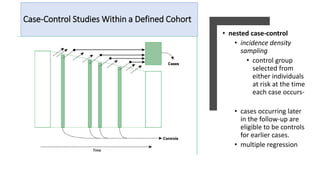
3) Cross-sectional studies examine exposure and outcome prevalence at a single point in time. Nested case-control studies select controls from the original cohort.