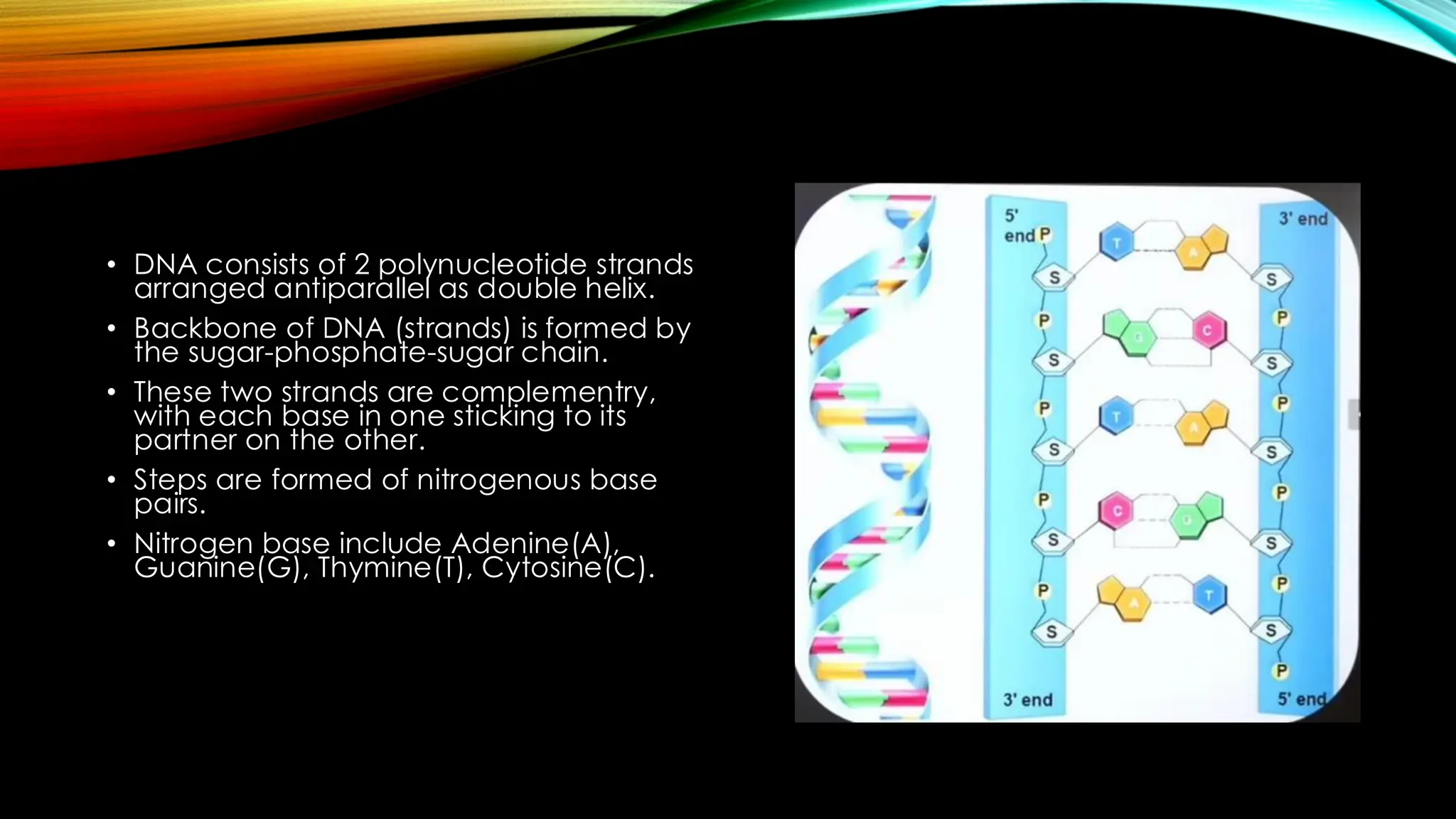
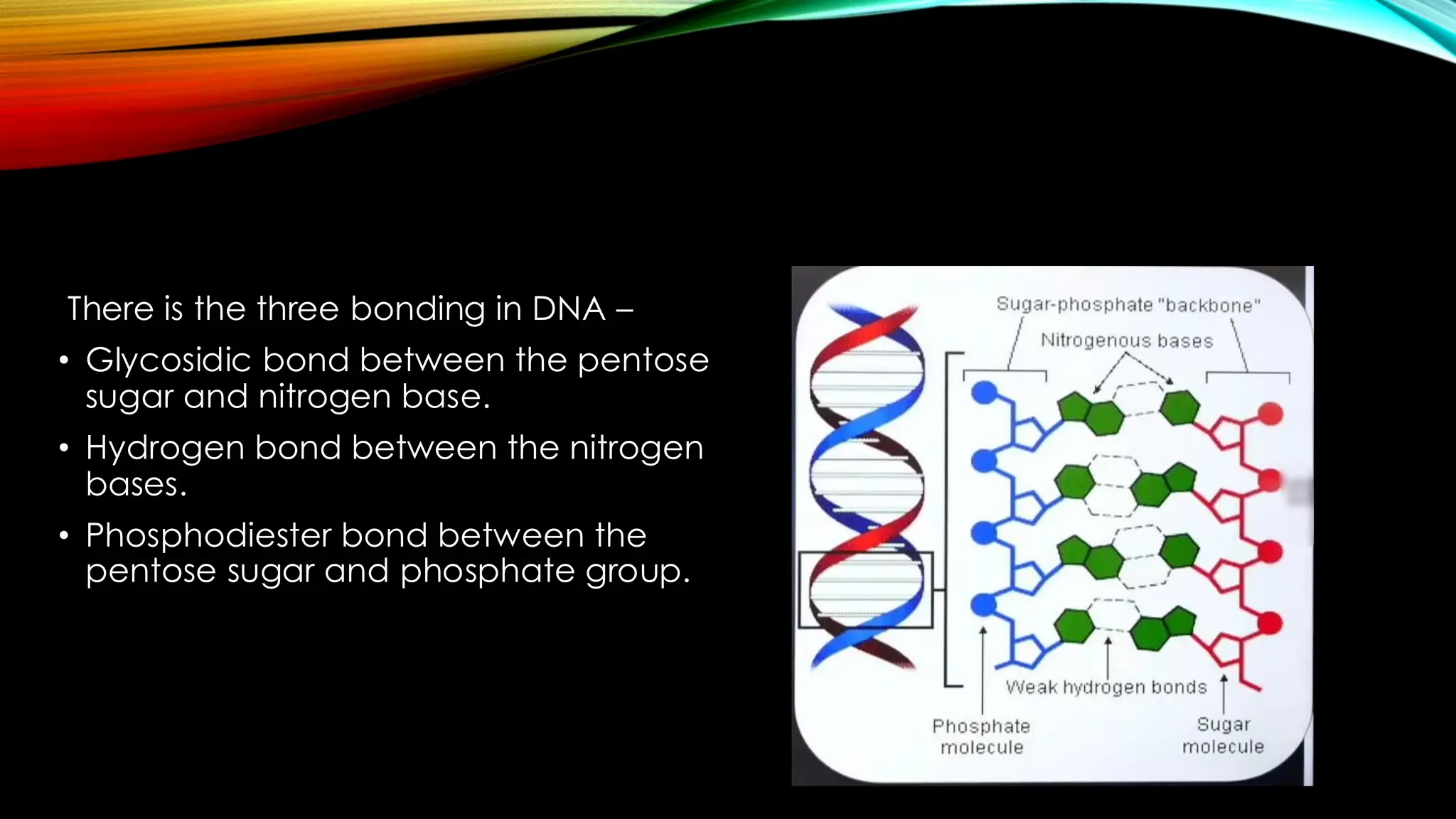
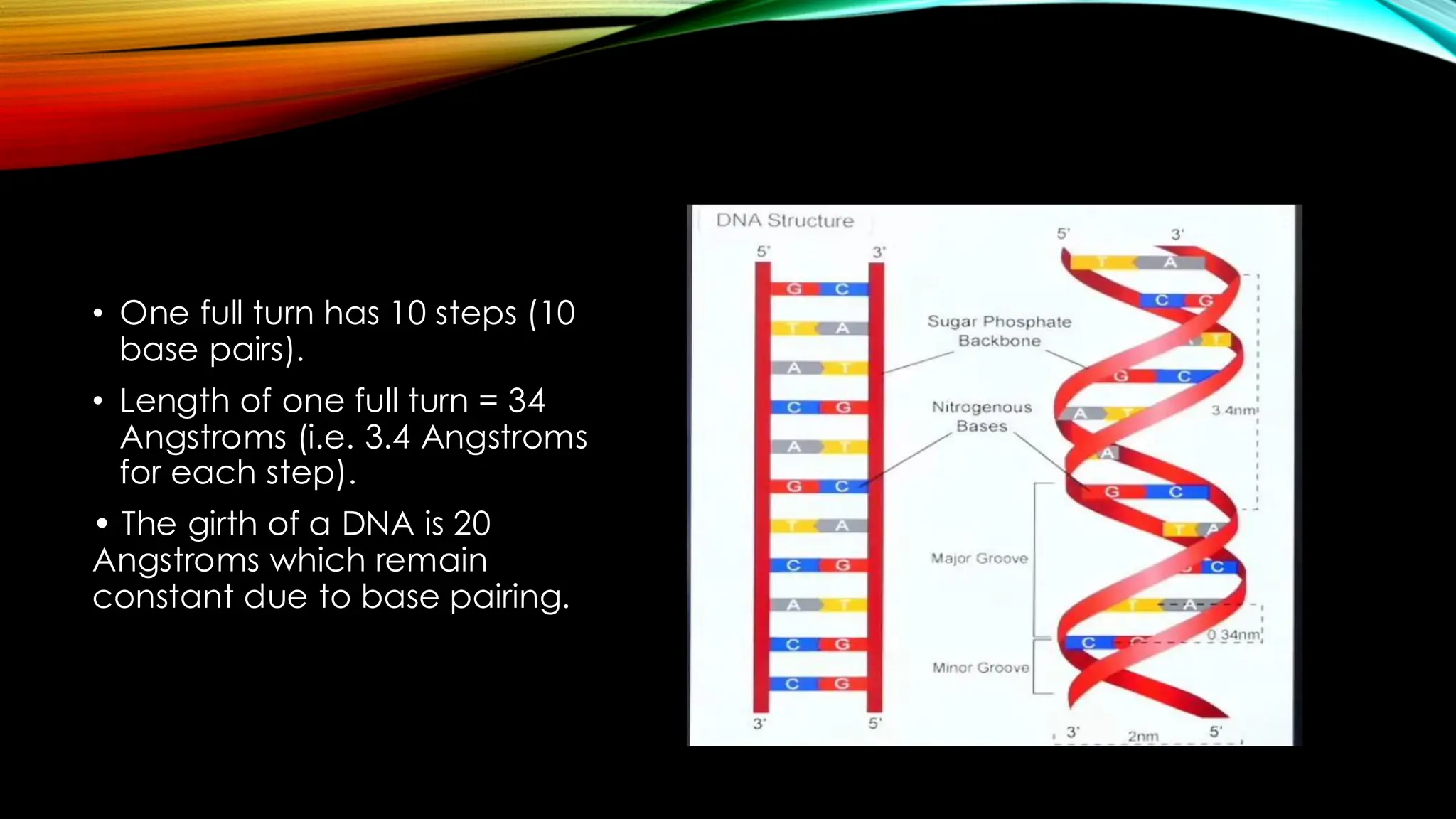
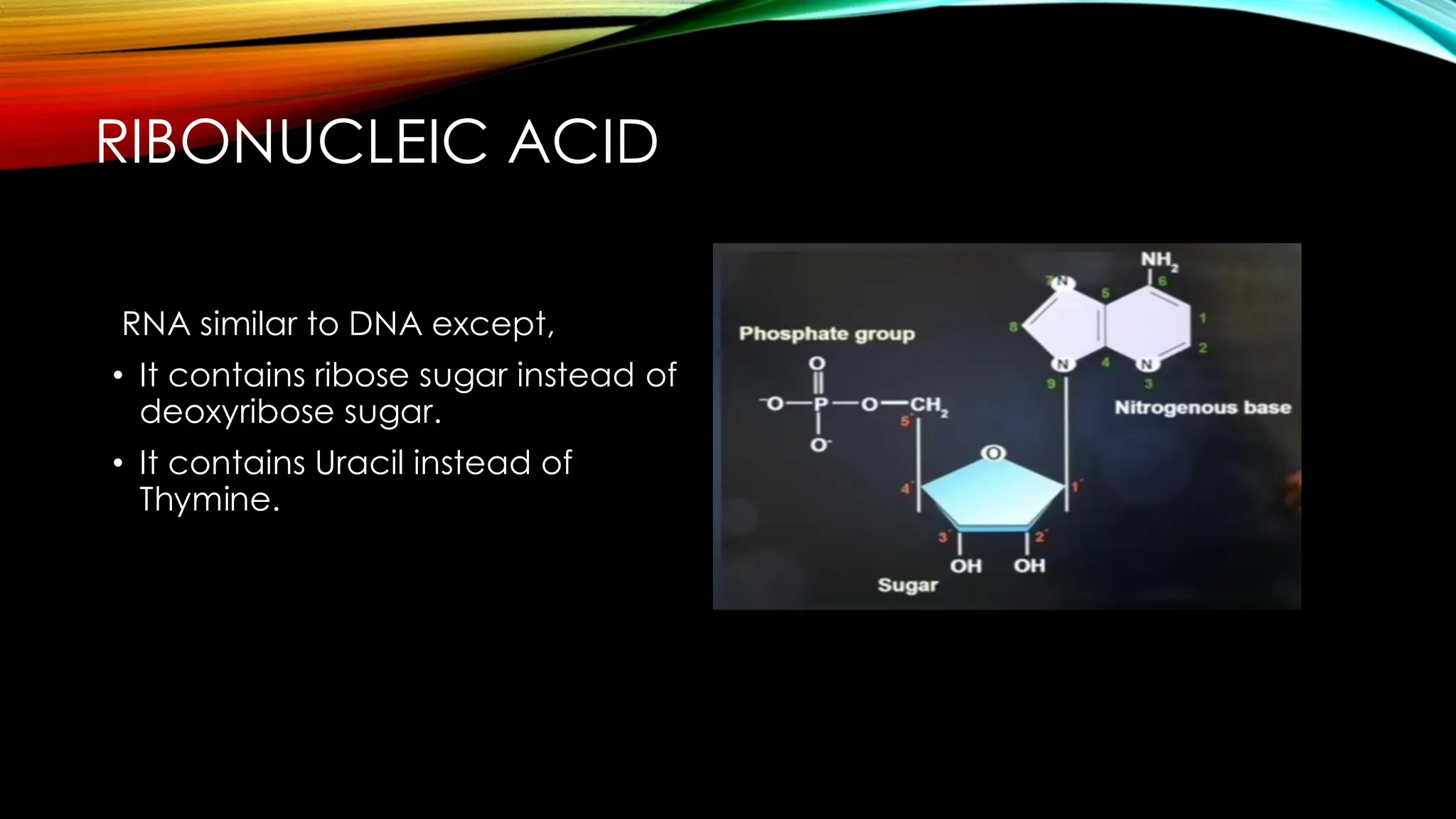
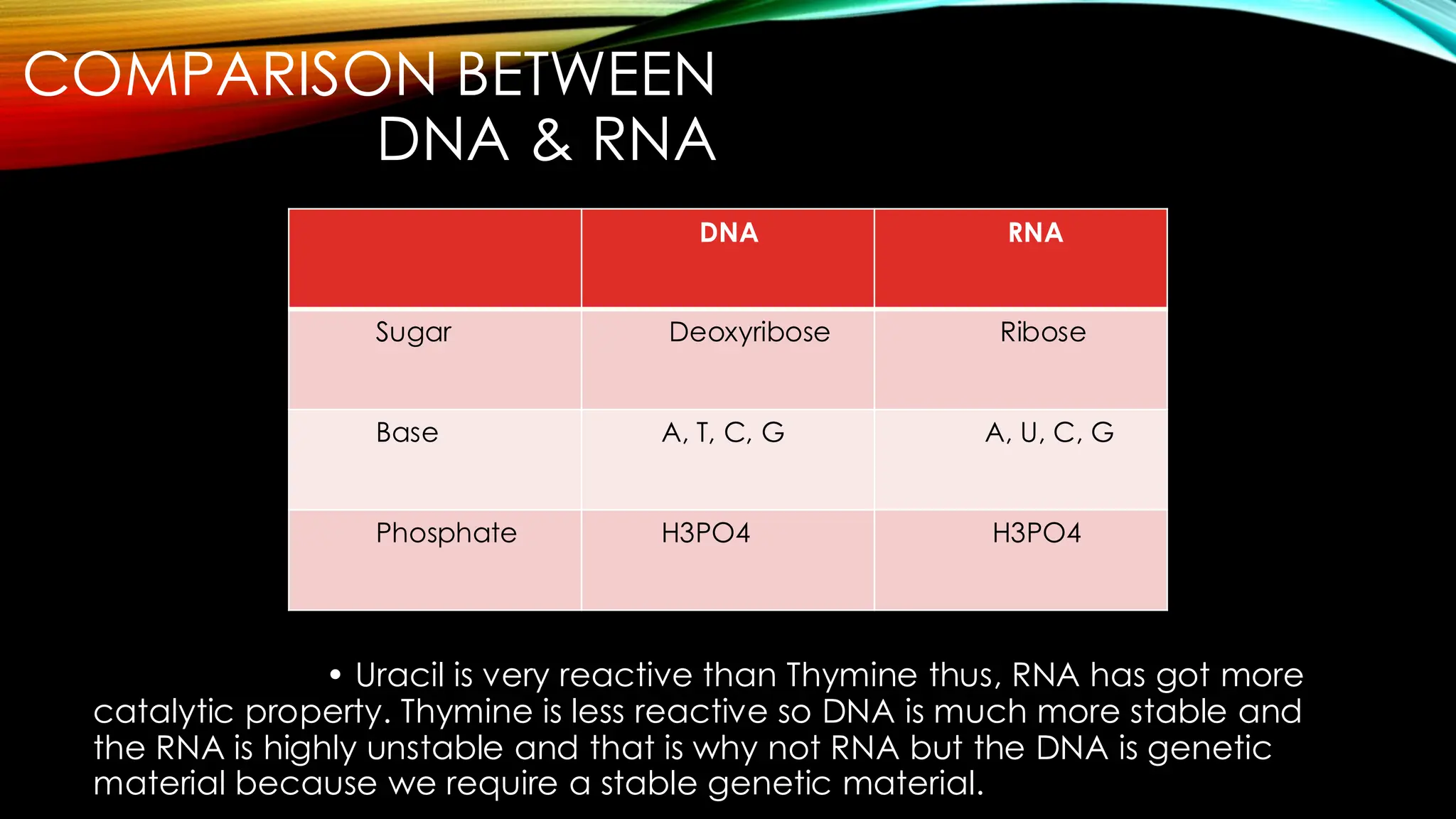
Nucleic acids, DNA and RNA, are essential information-carrying molecules of the cell, influencing protein synthesis and genetic inheritance. DNA is a double-stranded helix composed of deoxyribonucleotides, while RNA is typically single-stranded and includes ribonucleotides with uracil replacing thymine. The processes of transcription and the structural differences between RNA and DNA play critical roles in stabilizing genetic material and facilitating protein synthesis.