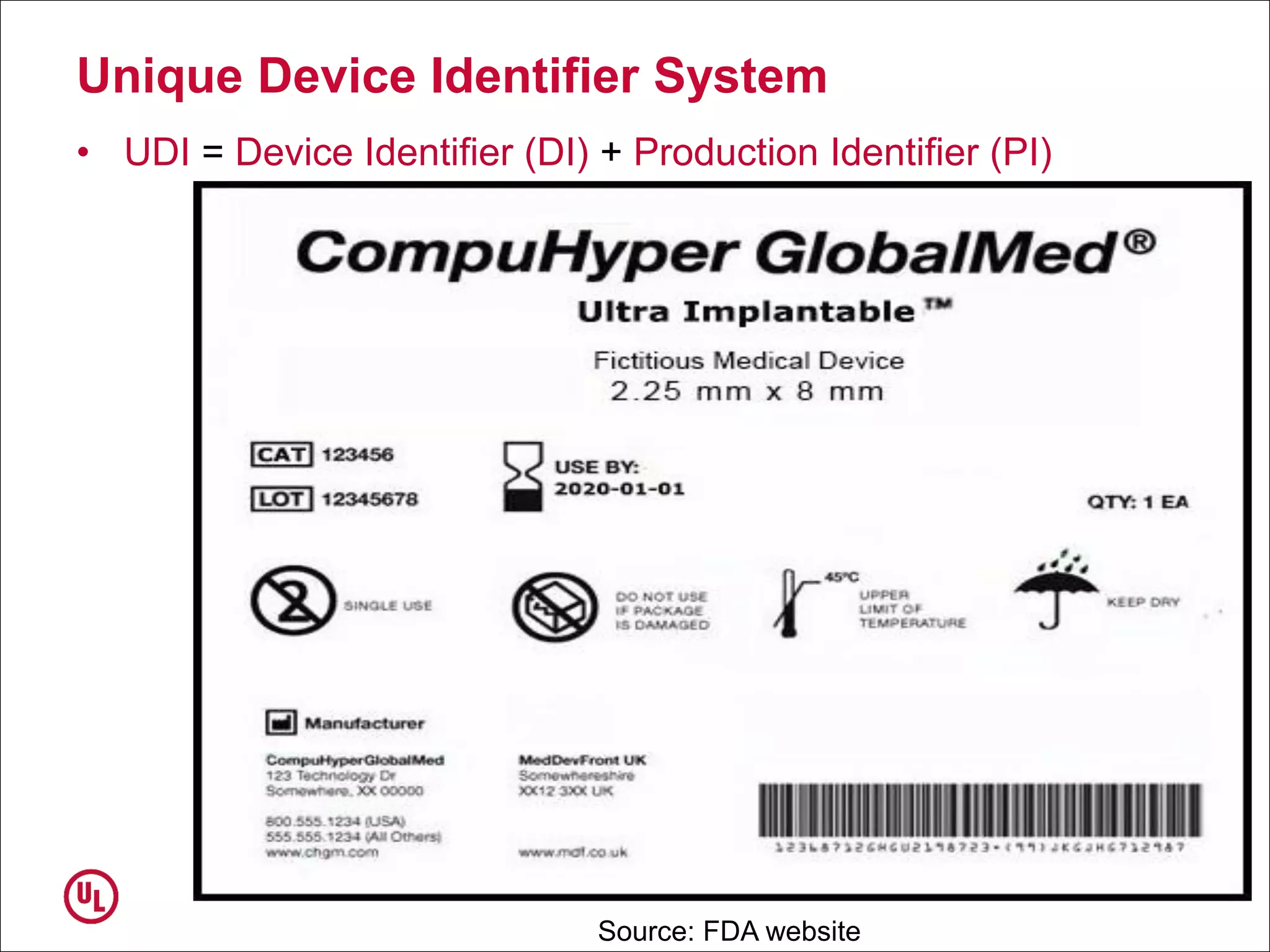
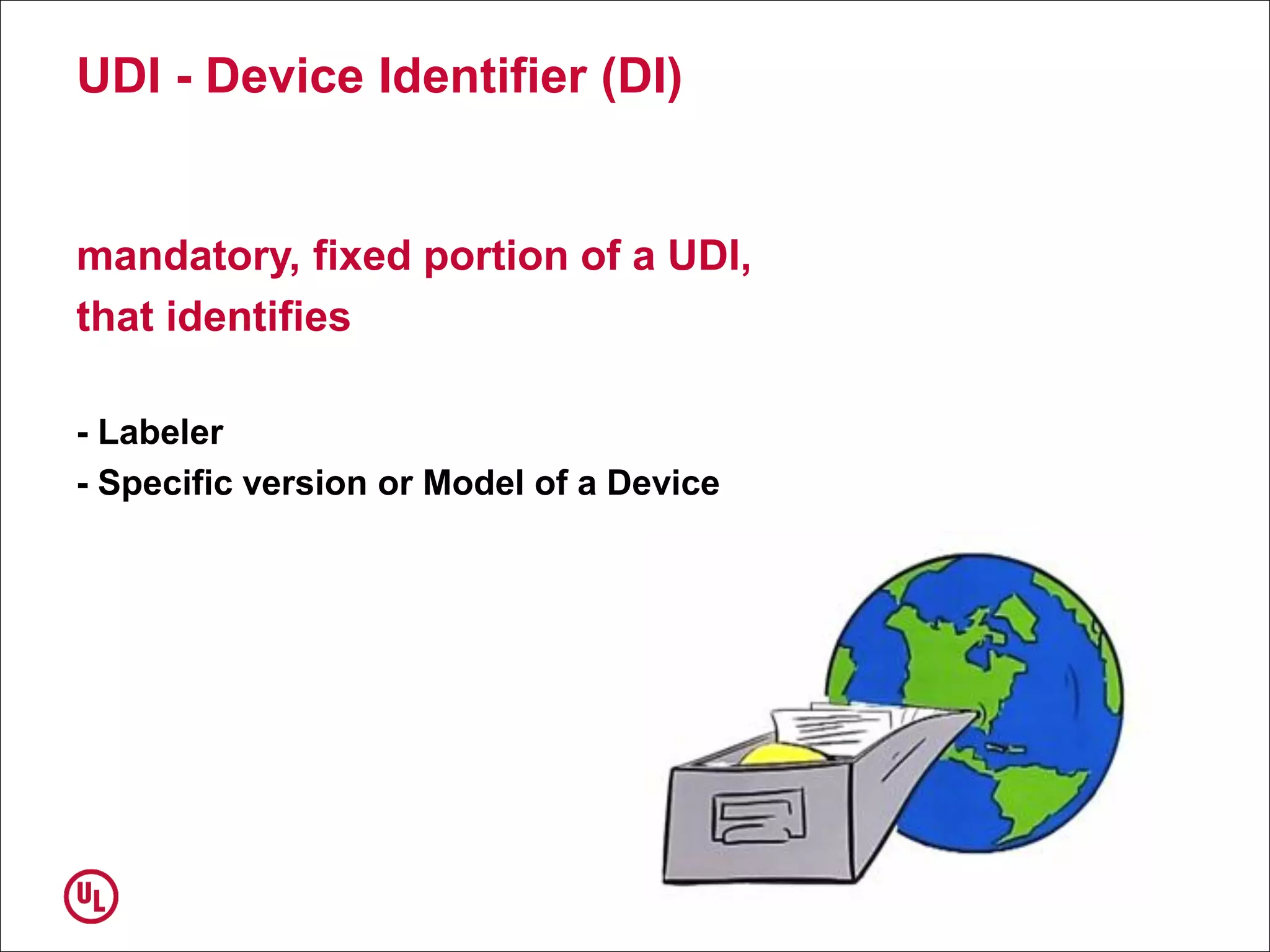
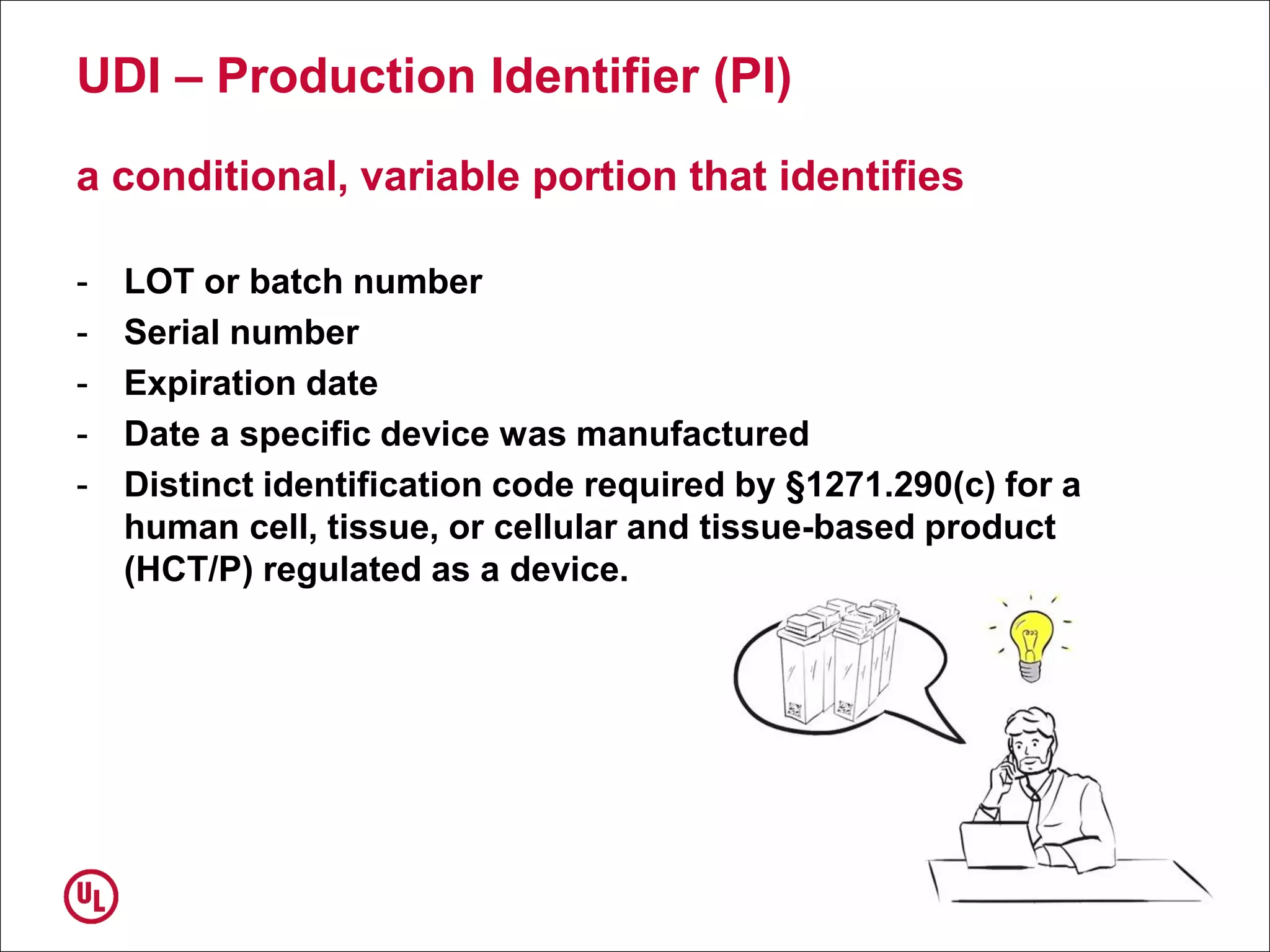
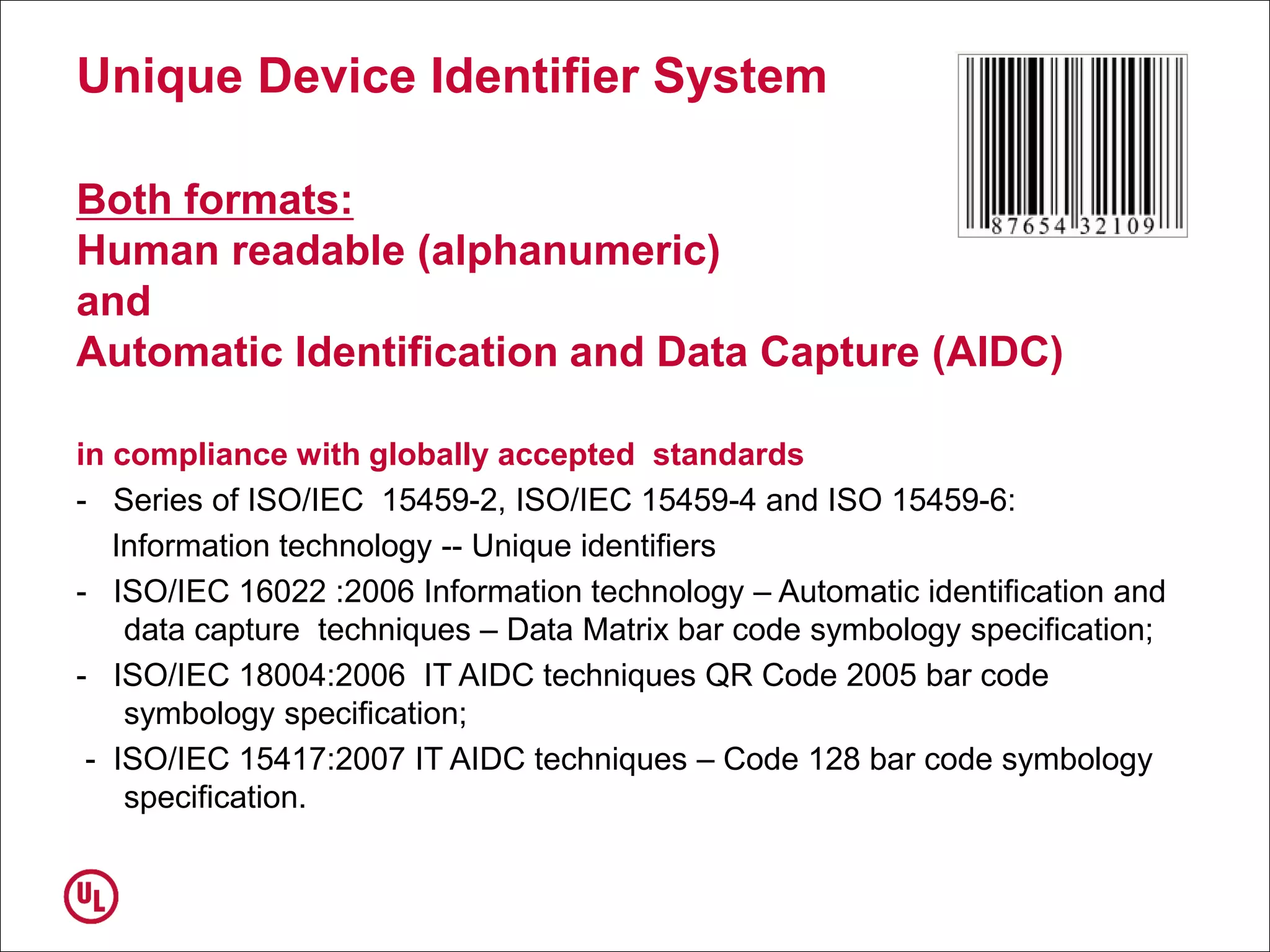
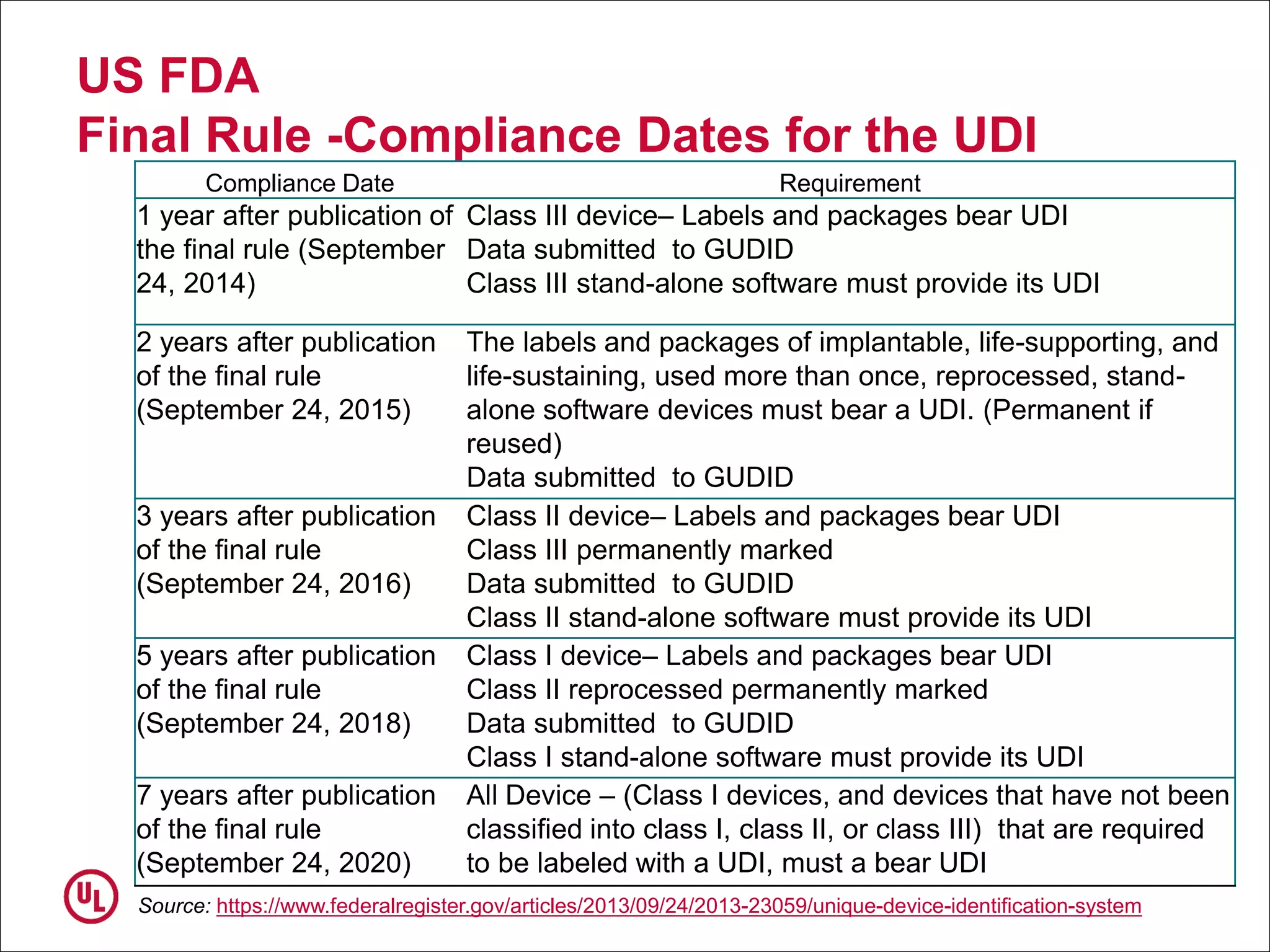
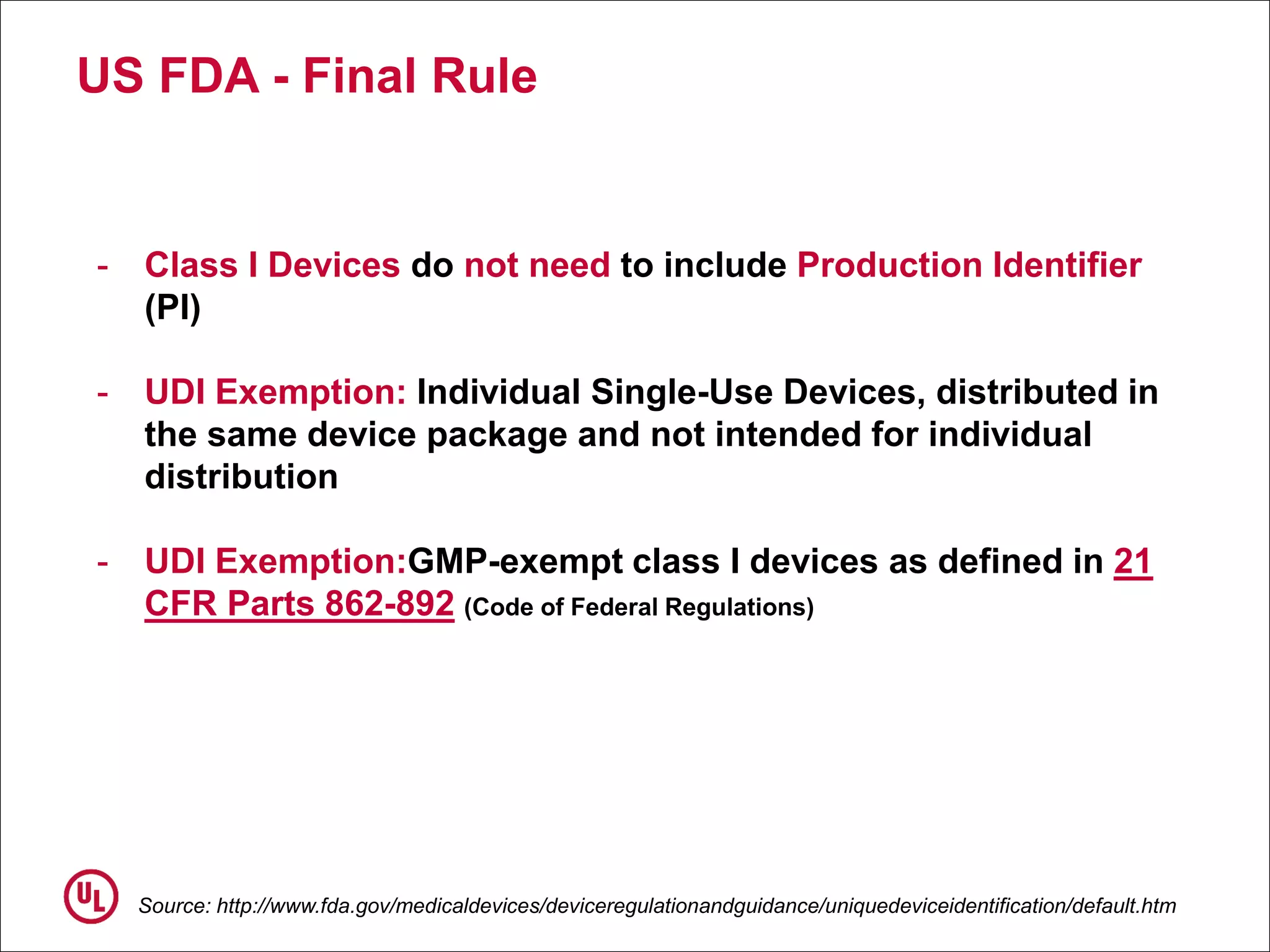
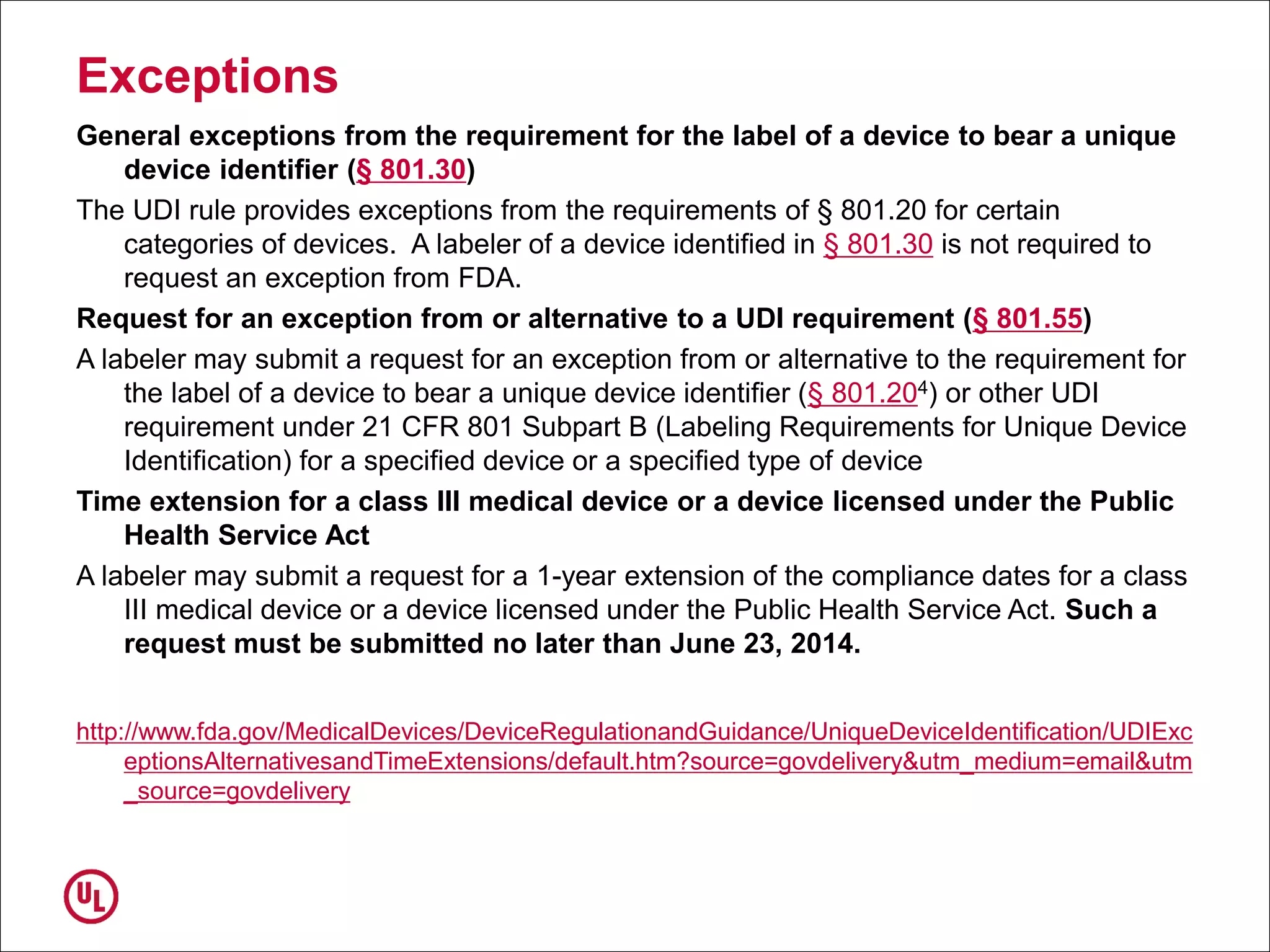
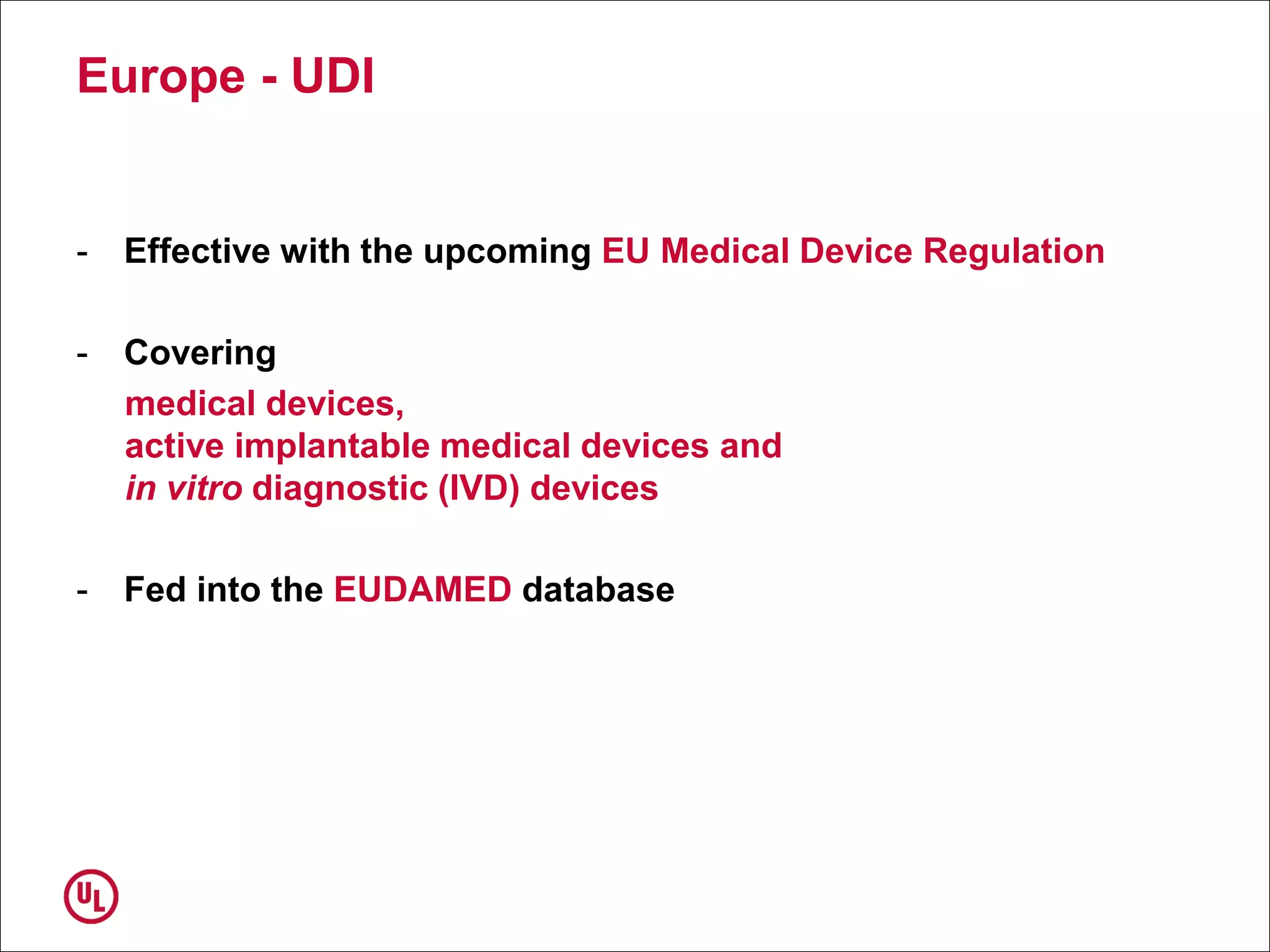
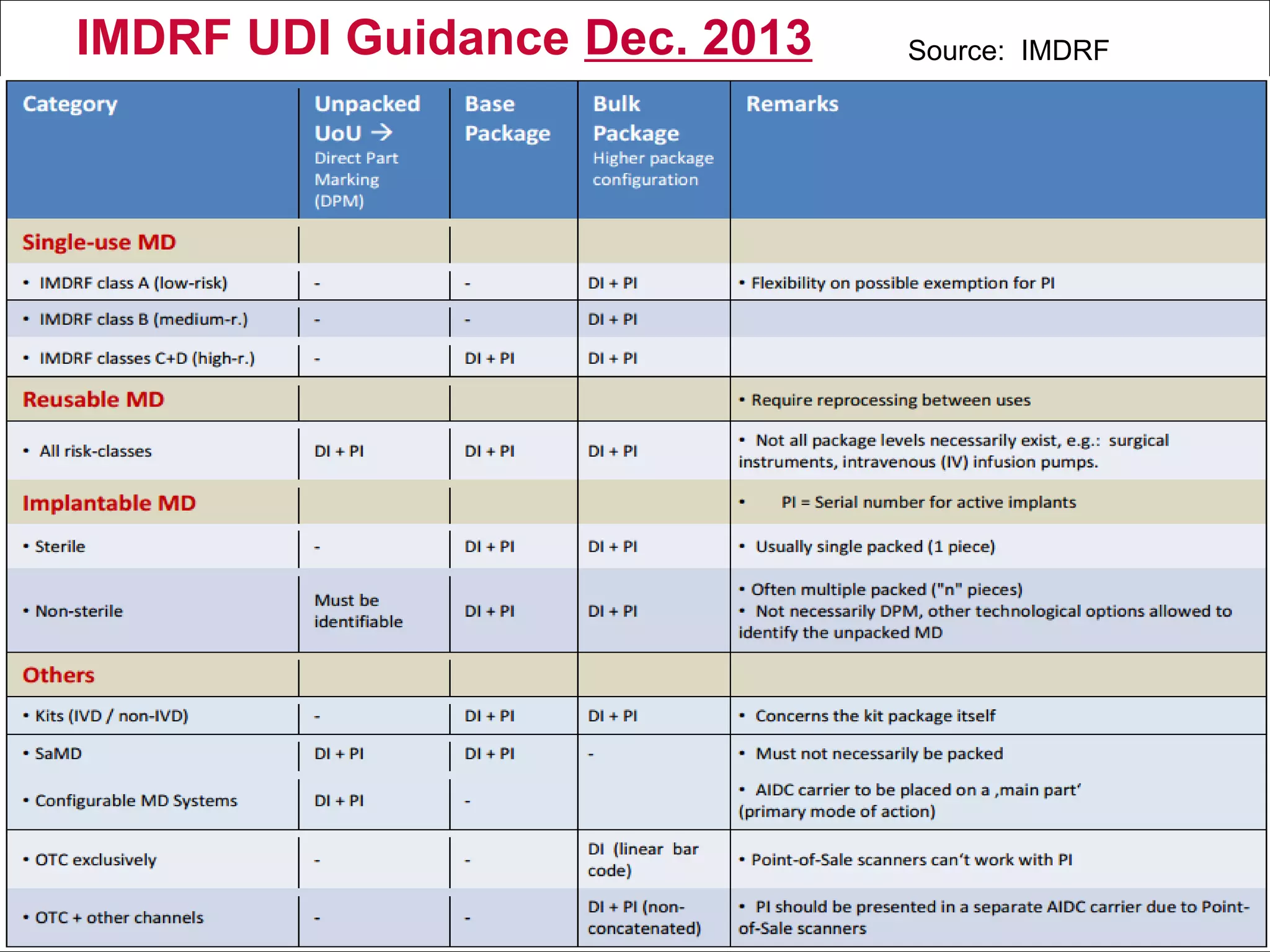
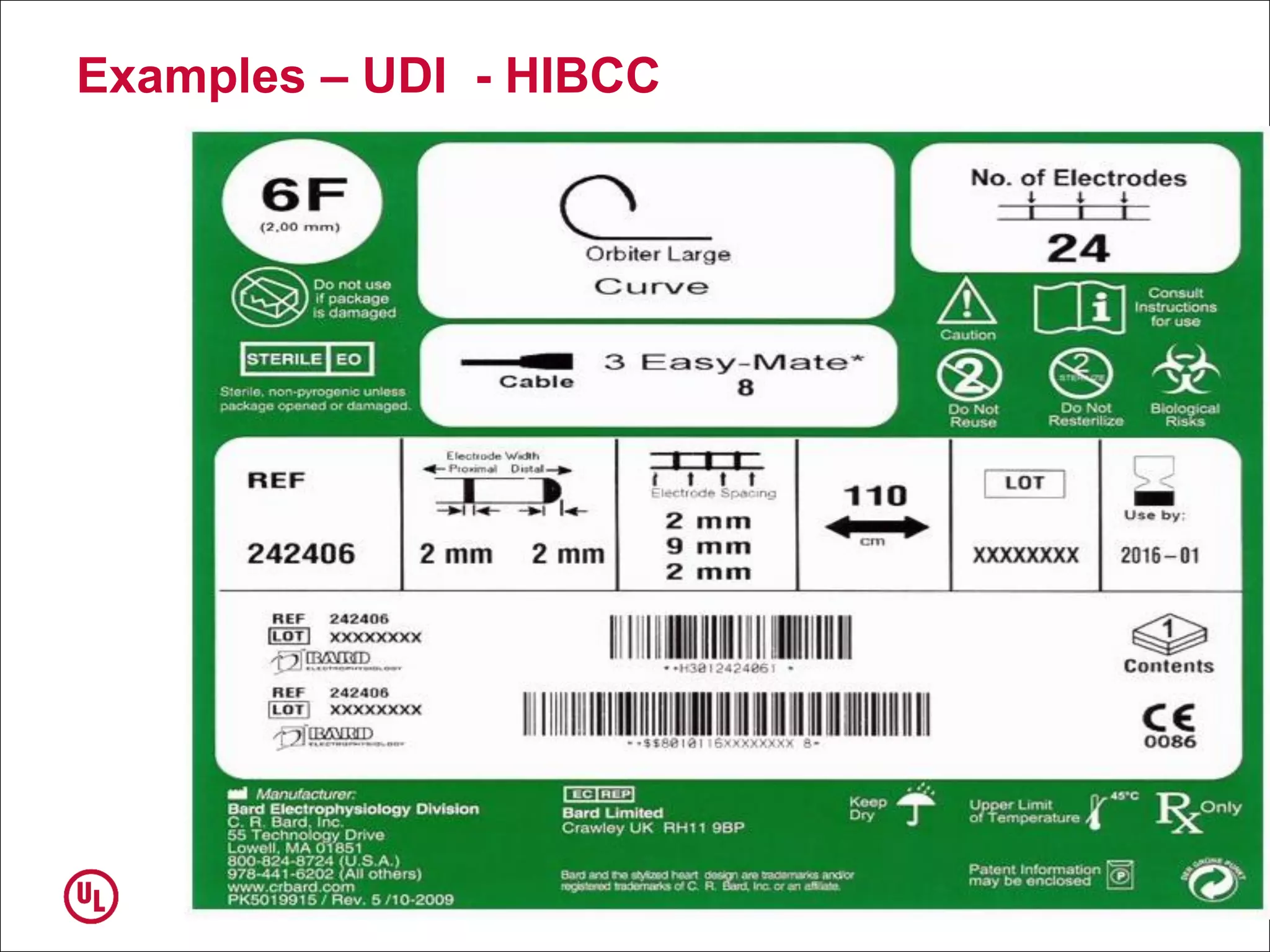
The document outlines the regulations and requirements surrounding the Unique Device Identifier (UDI) system mandated by the FDA to improve the tracking and identification of medical devices throughout their lifecycle. It details the background leading to the UDI rule, the benefits of traceability, compliance timelines, and the role of government in facilitating the implementation of the UDI system. Additionally, it discusses the exceptions and alternatives to UDI requirements and highlights the importance of a standardized identification system for patient safety and market surveillance.






![Regulations Affected by UDI Rule
PART 801—LABELING
Subpart A—[Amended]
Subpart B—Labeling Requirements for Unique Device Identification
PART 803—MEDICAL DEVICE REPORTING
PART 806—MEDICAL DEVICES; REPORTS OF CORRECTIONS AND
REMOVALS
PART 810—MEDICAL DEVICE RECALL AUTHORITY
PART 814—PREMARKET APPROVAL OF MEDICAL DEVICES
PART 820—QUALITY SYSTEM REGULATION
PART 821—MEDICAL DEVICE TRACKING REQUIREMENTS
PART 822—POSTMARKET SURVEILLANCE
7](https://image.slidesharecdn.com/2014ulfdaudistrategies-140603103344-phpapp01/75/Strategies-for-meeting-FDA-s-UDI-Rule-7-2048.jpg)