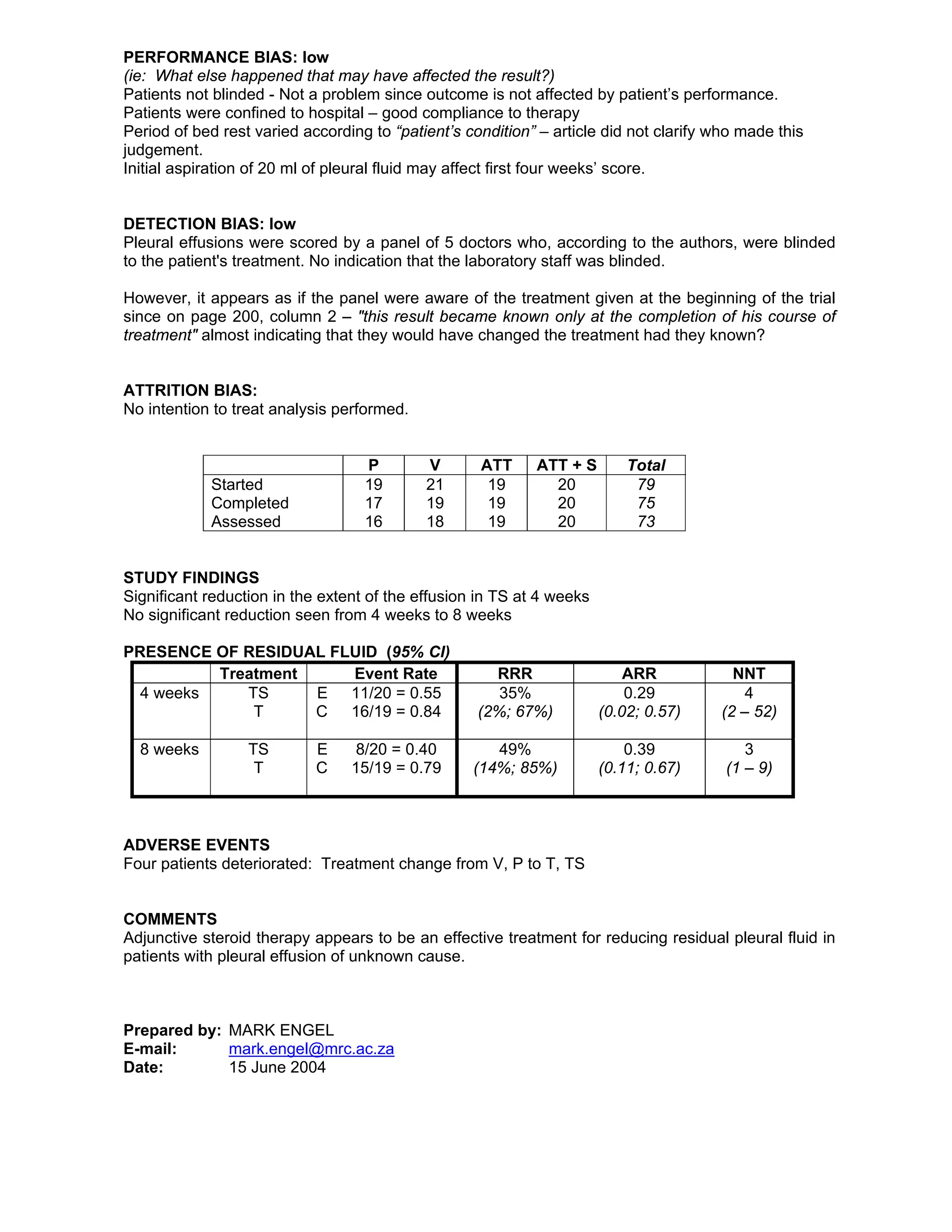
This randomized controlled trial evaluated the effectiveness of adjunctive steroid therapy for reducing residual pleural effusions in patients with tuberculosis (TB). The study involved 79 miners in South Africa with undiagnosed pleural effusions who were randomly assigned to standard antituberculosis treatment alone or with additional prednisolone therapy. Patients receiving additional steroid therapy had significantly lower rates of residual pleural effusion at both 4 weeks (55% vs 84%) and 8 weeks (40% vs 79%) of treatment. The results suggest that adjunctive steroid therapy is an effective treatment for reducing residual pleural fluid in patients with undiagnosed pleural effusions.