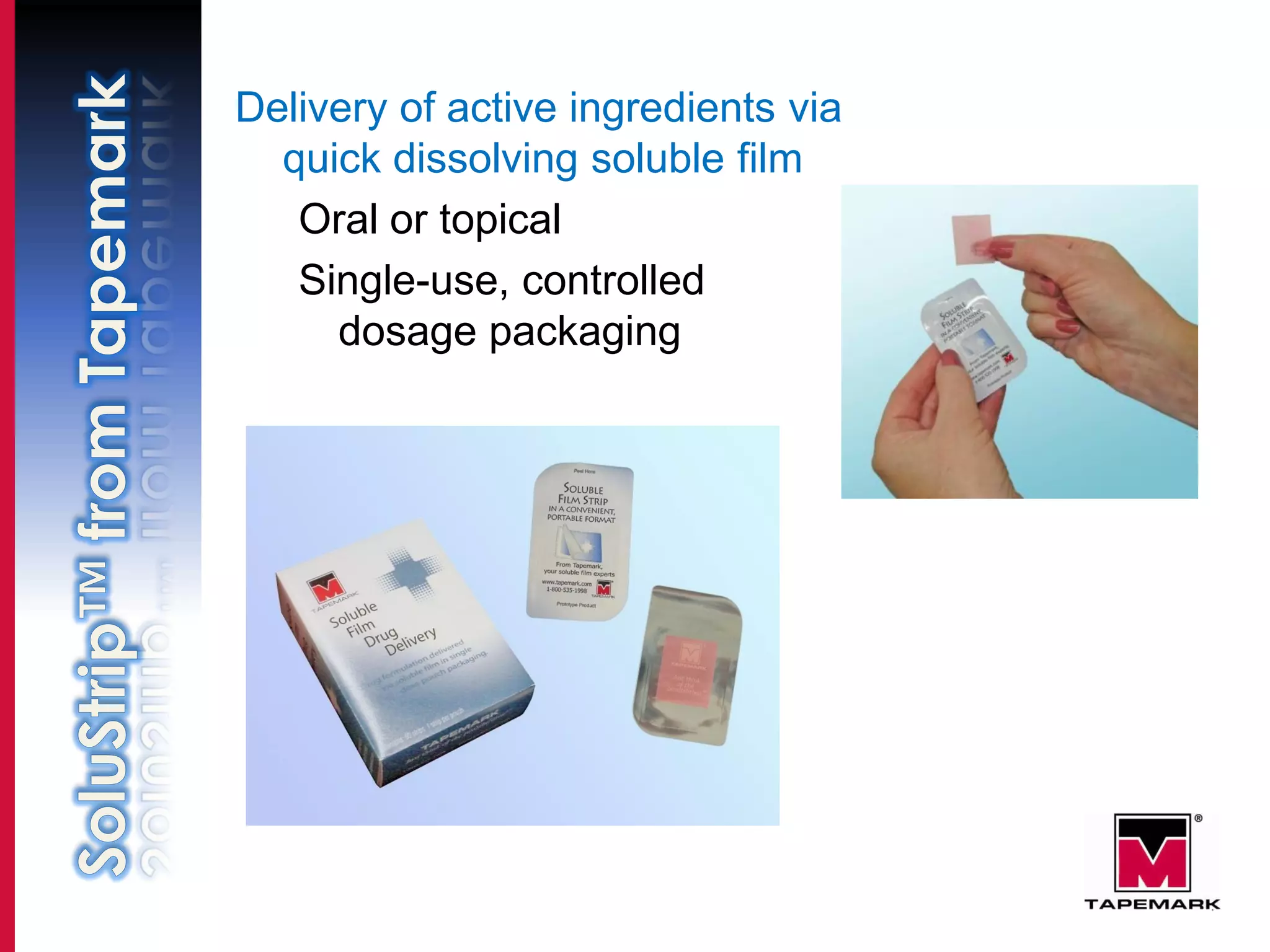
This document discusses using soluble film to deliver active ingredients through quick dissolving strips for oral or topical administration. It notes some key applications like tooth whitening, cough/cold relief, vitamins, and homeopathic remedies. It highlights benefits like ease of use, improved compliance, and diverse routes of entry. It also discusses partnerships with film suppliers, over 10 years of experience in film development and packaging, and regulatory compliance with FDA, EU, Japan, Australia, and other international standards.