Sodium Borohydride Poster
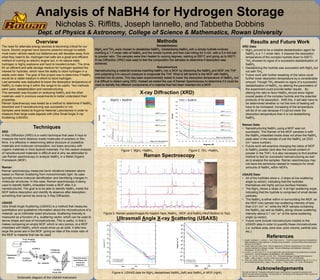
- 1. X-ray Diffraction (XRD) Figure 1. MgH2 +NaBH4. Figure 2. TiH2 +NaBH4. Raman Spectroscopy Figure 3. Raman peaks/images for Kapton Tape, NaBH4 , MOF, and NaBH4+MoF(Bottom to Top). Ultrasmall Angle X-ray Scattering (USAXS) Figure 4. USAXS data for MgH2 destabilized NaBH4 (left) and NaBH4 in MOF (right). Techniques XRD X-Ray Diffraction (XRD) is a useful technique that uses X-rays to measure the bond distance inside molecules of powders or thin films. It is effective in determining lattice structure of crystalline materials and molecular composition, but loses accuracy with organic materials or thick layered materials. For this reason analysis of nanostructured materials is difficult and is why we attempted to use Raman spectroscopy to analyze NaBH4 in a Metal Organic Framework (MOF). Raman Raman spectroscopy measures bond vibrations between atoms based on Raman Scattering from monochromatic light. Its uses typically involve molecule identification and identifying changes in molecular structures. In this case, Raman spectroscopy is being used to identify NaBH4 imbedded inside a MOF after it is nanostructured. The goal is to be able to identify NaBH4 inside the MOF before desorption and identify its absence after desorption, something that cannot be done by X-Ray Diffraction. USAXS Ultra Small Angle Scattering (USAXS) is a method that measures quantitative and qualitative information about the microstructure of a material, up to millimeter sized structures. Scattering intensity is measured as a function of q, scattering vector, which can be used to derive shape and size of microstructures. This is useful, in that it makes comparing an empty MOF, which is very porous, to a MOF imbedded with NaBH4 which would show up as solid. It tells how large the pores are in the MOF, giving an idea of the molar ratio of the MOF to material that can be used. Schematic diagram of the USAXS instrument Overview The need for alternate energy sources is becoming critical for our future. Electric engines have become powerful enough to satisfy most motor vehicle needs but batteries are still decades away from what they need to be. Hydrogen fuel cells are a great and efficient method of running an electric engine but, in its natural state, hydrogen is highly explosive and hard to transfer/contain. The drive for an efficient and safe storage medium for hydrogen sparked the interest for hydrides, which have the ability to store hydrogen in a stable solid state. The goal of this project was to determine if NaBH4 would be a viable medium in which to store hydrogen. Last semester was dedicated to lower the desorption temperature of NaBH4 to make bring it within the range to be useful. Two methods were used, destabilization and nanostructuring. This semester was focused on analyzing NaBH4 and the other materials used in previous experiments to better understand their properties. Raman Spectroscopy was tested as a method to determine if NaBH4 desorbed and if nanostructuring was successful or not. Samples were tested at Argonne National Laboratories in order to measure their large scale aspects with Ultra Small Angle X-ray Scattering (USAXS). Results and Future Work XRD Data: • MgH2 proved to be a reliable destabilization agent for NaBH4 in a 1:1 molar ratio. It lowered the desorption temperature of the complex hydride down to 500°C. TiH2 showed no signs of a successful destabilization of NaBH4. • Destabilizing the hydride was successful with MgH2 but not with TiH2. • Future work with further tweaking of the ratios could further lower desorption temperature by a considerable amount. Though TiH2 showed no signs of a successful destabilization of NaBH4, by altering the parameters of the experiment could provide better results. . By altering the ratio to favor NaBH4 should show higher overall peaks of the complex hydride and the potential products of its desorption. With this, it will later have to be determined whether or not the time of heating will have to be increased. Increasing of the temperature will be of no use because if it cannot lower the desorption temperature than it is not destabilizing NaBH4. Raman Data: • Nanostructuring NaBH4 using a MOF was not successful. The Raman of the MOF samples in with the NaBH4 imbedded inside does not show the NaBH4 peak seen in the sample of just the NaBH4 (at 2333 cm-1 wave number). • Future work will examine changing the ratios of MOF to NaBH4 powder (and also the overall content of powder in the THF). It is also necessary to find another method to test for successful nanostructuring as well as to analyze the samples. Raman spectroscopy may not have the sensitivity needed to measure for small amounts of NaBH4 within MOFs. USAXS Data: • All of the hydrides show a -3 slope at low scattering angle (q-vector), indicating that the hydrides themselves are highly porous (surface fractals). • The MgH2 shows a slope of -4 at high scattering angle, indicating that the hydride is comprised of small dense particles. • The NaBH4 is either within or surrounding the MOF, as the MOF-only sample has scattering intensity of less than 0.01 cm-1 sr1 while the MOF sample containing the NaBH4 (as well as NaBH4 along) shows scattering intensity above 0.1 cm-1 sr1 at the same scattering angle (q-vector). • Future work include microstructure models to the USAXS data in order to quantify these microstructures (i.e. surface area, pore size, pore volume, particle size, etc.). References 1. Alapati, Sudhakar V., Johnson, J. Karl, Sholl, David S., “First Principals Screening of Destabilized Metal Hydrides for High Capacity H2 Storage using Scandium”, Journal of Alloys and Compounds, 446-447, 23-27(2007). 2. Daniel, Reed and David, Book. “Recent applications of Raman spectroscopy to the study of complex hydrides for hydrogen storage.” School of Metallurgy and Materials, University of Birmingham. 15 (2011) 62–72. 3. Levine, L. E. and Long, G. G. “X-ray imaging with ultra-small-angle X-ray scattering as a contrast mechanism.” Journal of Applied Crystallography. 0021-8898(2004). 4. Mao, J.F., Yu X.B., Guo Z.P., Liu H.K., Ni J., “Enhanced Hydrogen Storage Performances of NaBH4- - MgH2 System”, Journal of Alloys and Compounds, 479, 619-623(2009). 5. Yang, Jun, Sudik, Andrea, Wolverton, C., “Destabilizing LiBH4 with a Metal( M= Mg, Al, Ti, V, Cr, or Sc) or Metal Hydride (MH2 = MgH2, TiH2, or CaH2”, J. Phys. Chem., 111, 19134-19140(2007). Acknowledgements We wish to thank Mr. Christopher Bennett for help in learning how to operate the glovebox and perform the ball milling of hydrides. We also thank Dr. Jan Ilavsky at the Advanced Photon Source for help in measuring our USAXS data. Analysis of NaBH4 for Hydrogen Storage Nicholas S. Riffitts, Joseph Iannello, and Tabbetha Dobbins Dept. of Physics & Astronomy, College of Science & Mathematics, Rowan University Methods Destabilization MgH2 and TiH2 were chosen to destabilize NaBH4. Destabilizing NaBH4 with a simple hydride involves combing a 1:1 molar ratio of NaBH4 and the simple hydride and then ball milling for 5 min. with a 5:4 mill ball to powder ratio. The samples were then heated to varying degrees, starting at 500°C and going up to 650°C. X-ray Diffraction (XRD) was used to test the composition the samples to determine if desorption was achieved. Nanostructure Nanostructuring a material involves inserting NaBH4 into a MOF by dissolving the NaBH4 and MOF into THF and subjecting it to vacuum pressure to evaporate the THF. What is left behind is the MOF with NaBH4 inserted into its pores. This has been experimentally tested to lower the desorption temperature of NaBH4, but it is difficult to detect using XRD. Instead we tested the use of Raman Spectroscopy to determine if it could be used to identify the different components of a material that had been inserted into a MOF.