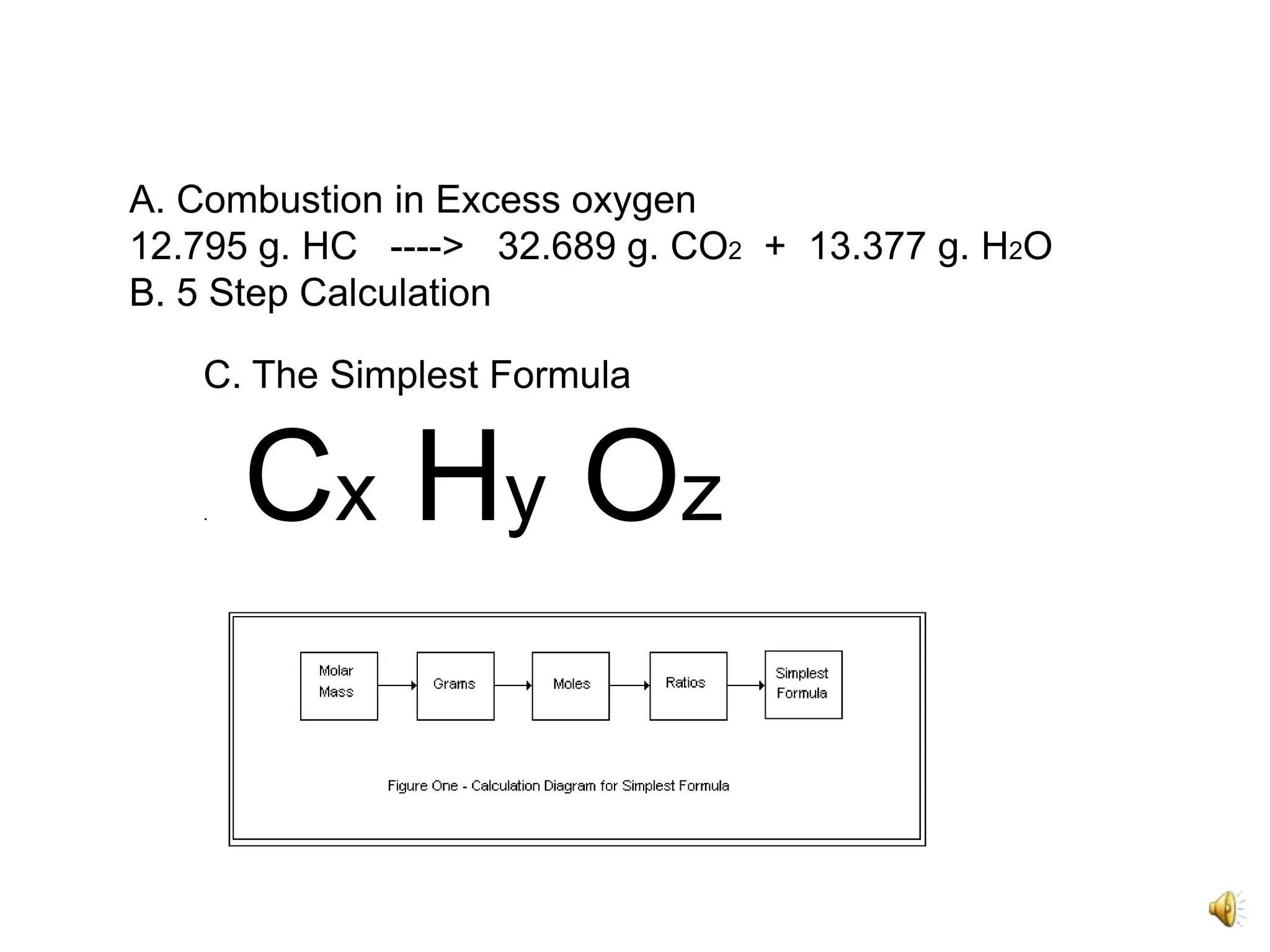
1) The document describes the simplest formula calculation for combustion of hydrocarbons in excess oxygen.
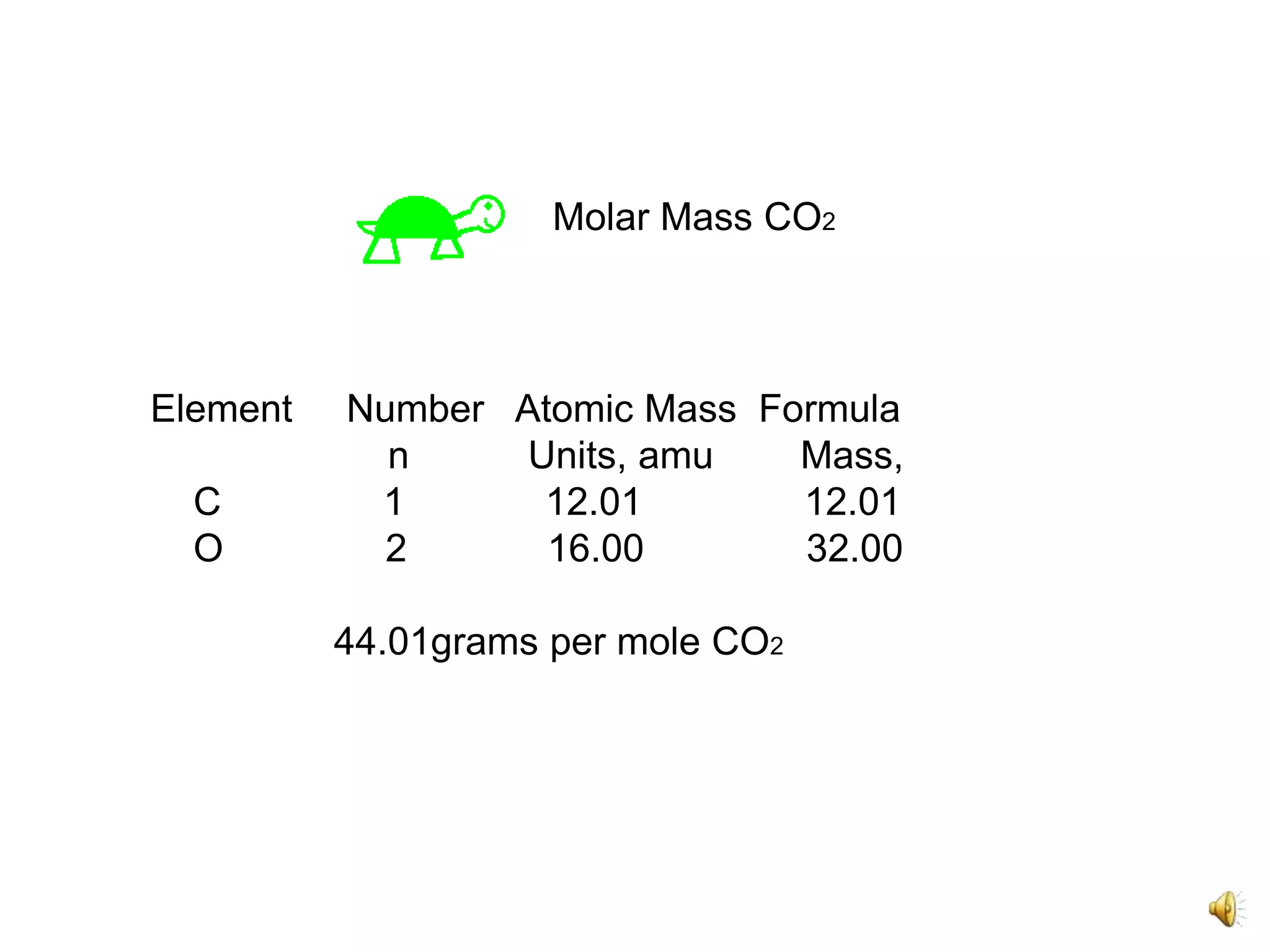
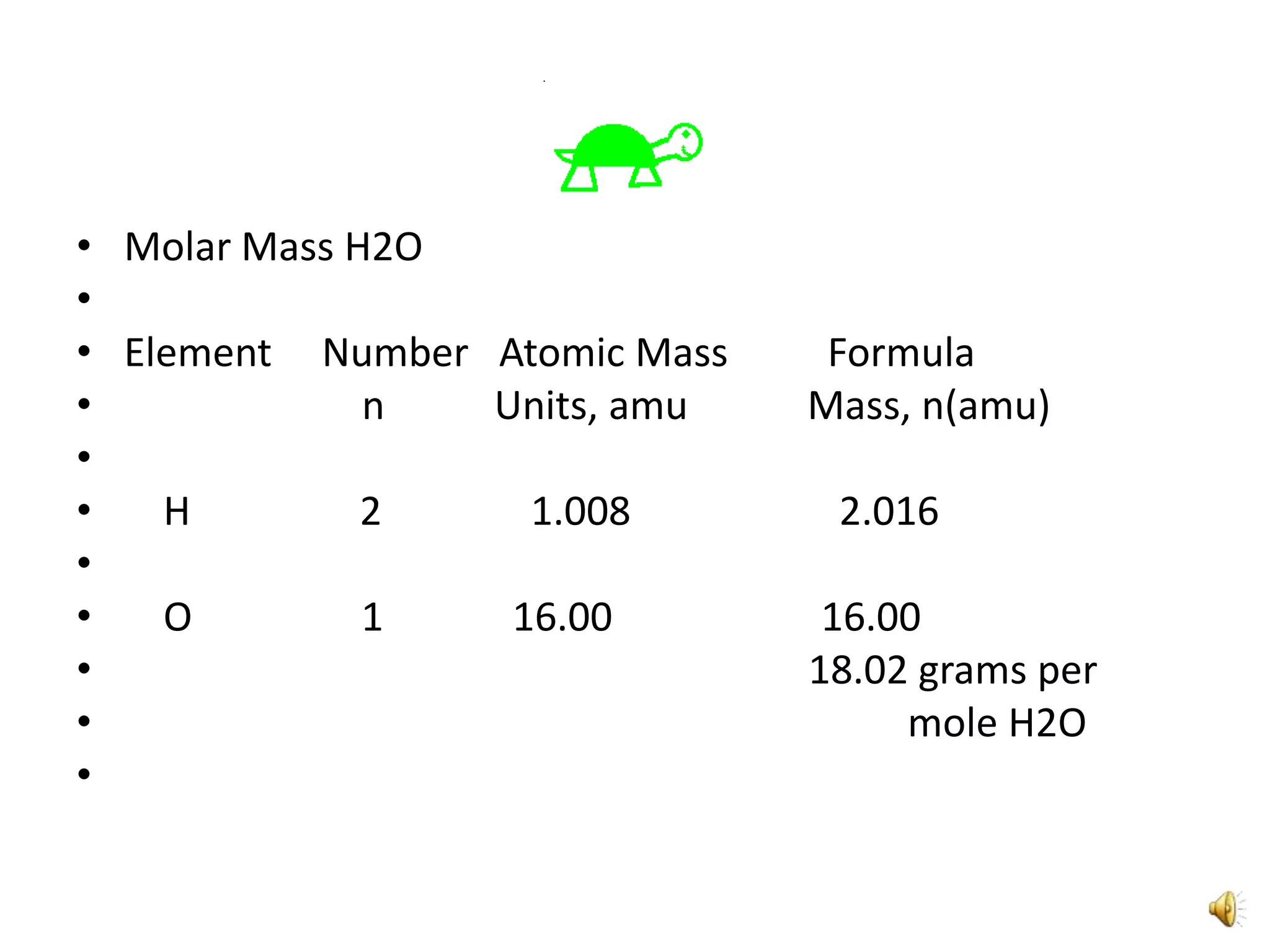
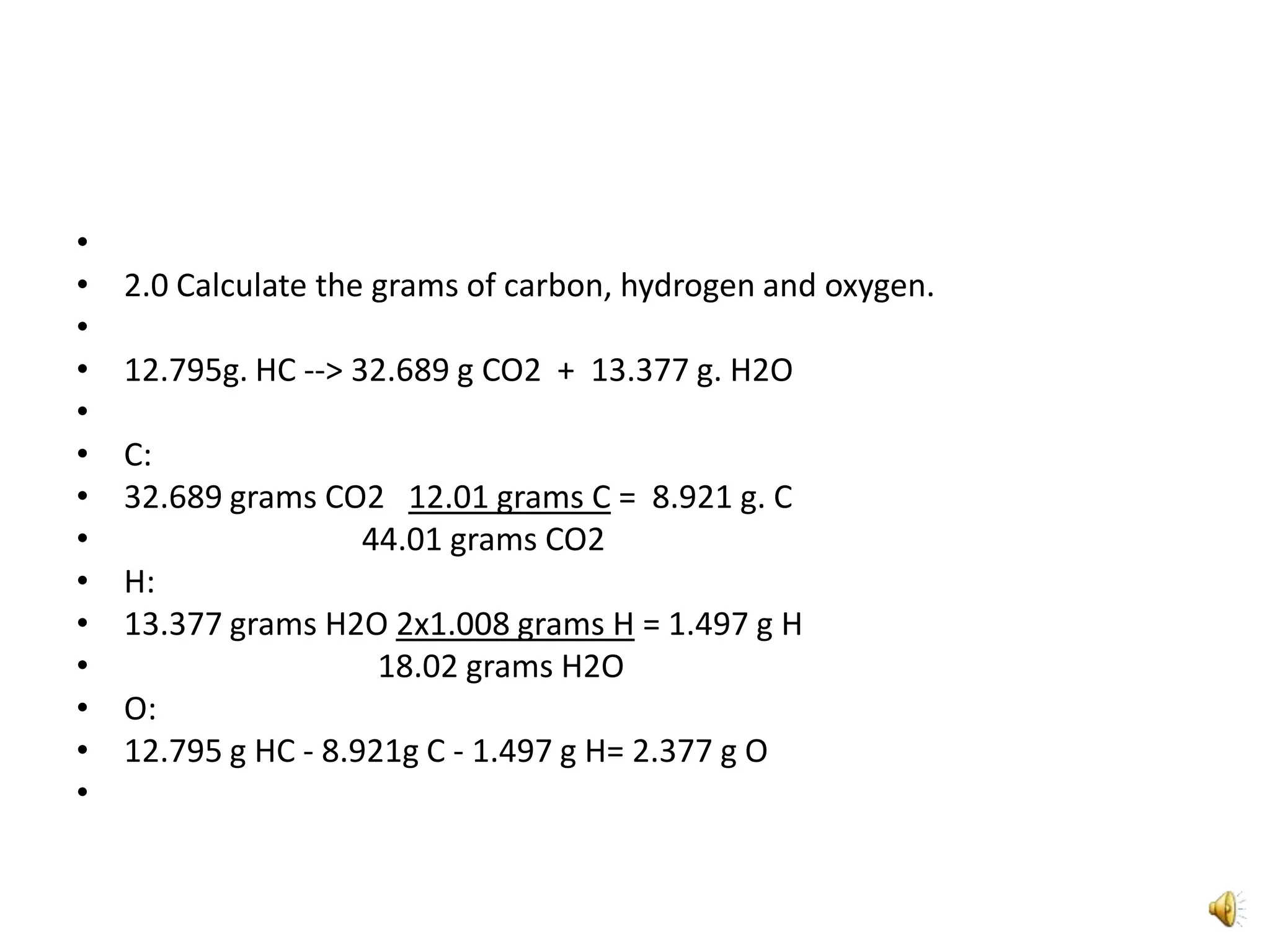
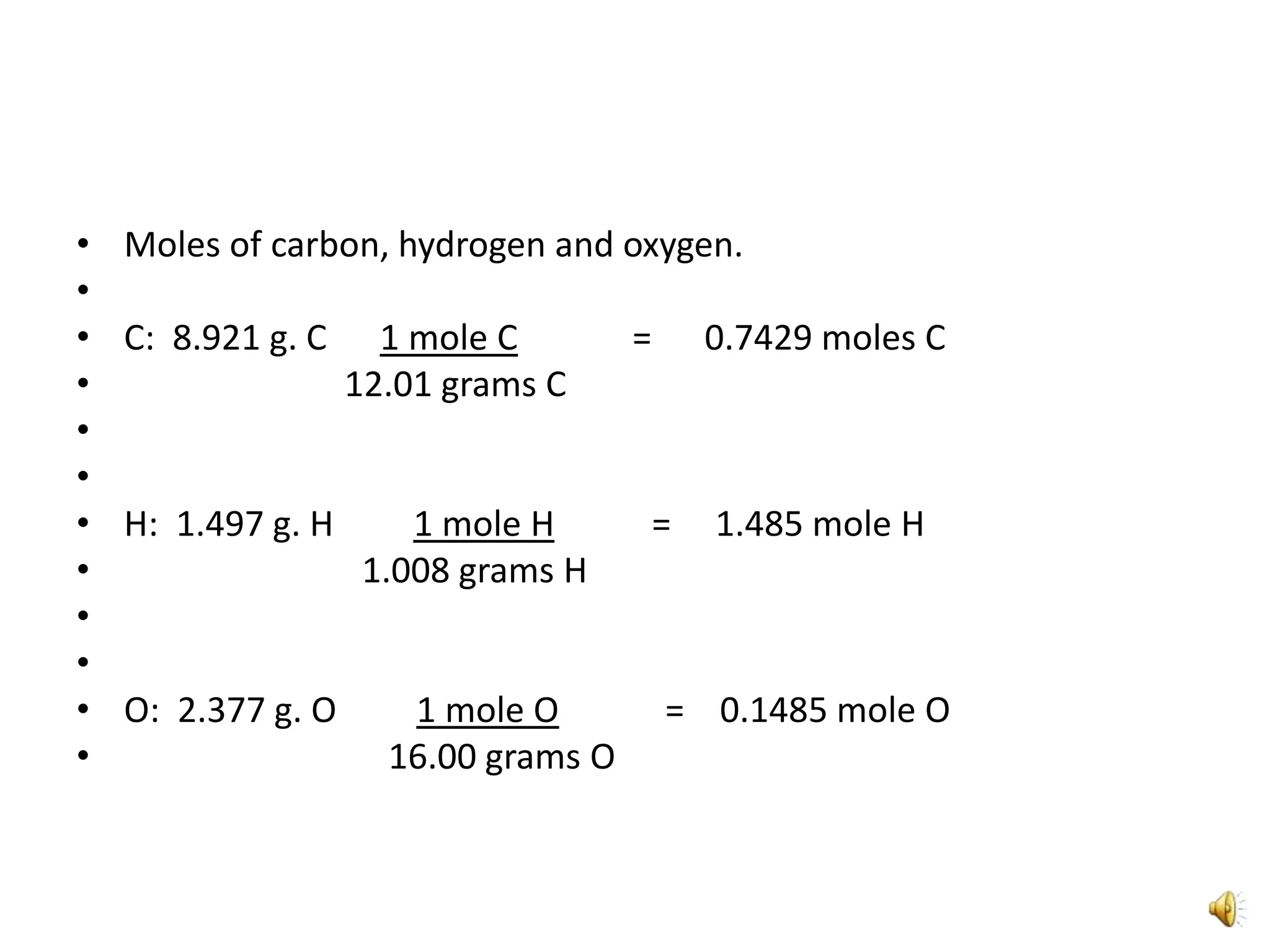
2) It involves a 5 step process of calculating the grams of carbon, hydrogen, and oxygen based on the combustion reaction and determining the moles of each element.
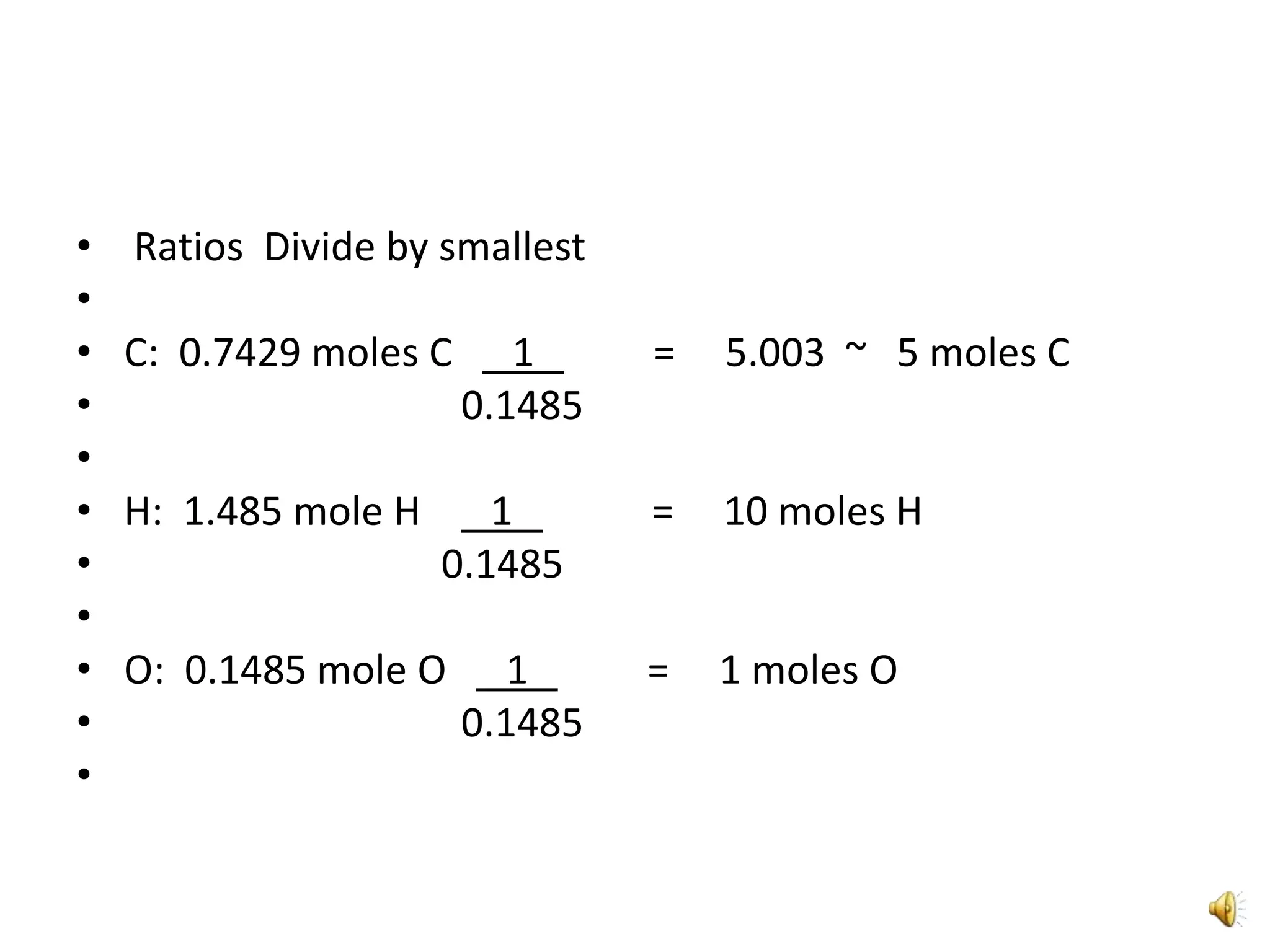
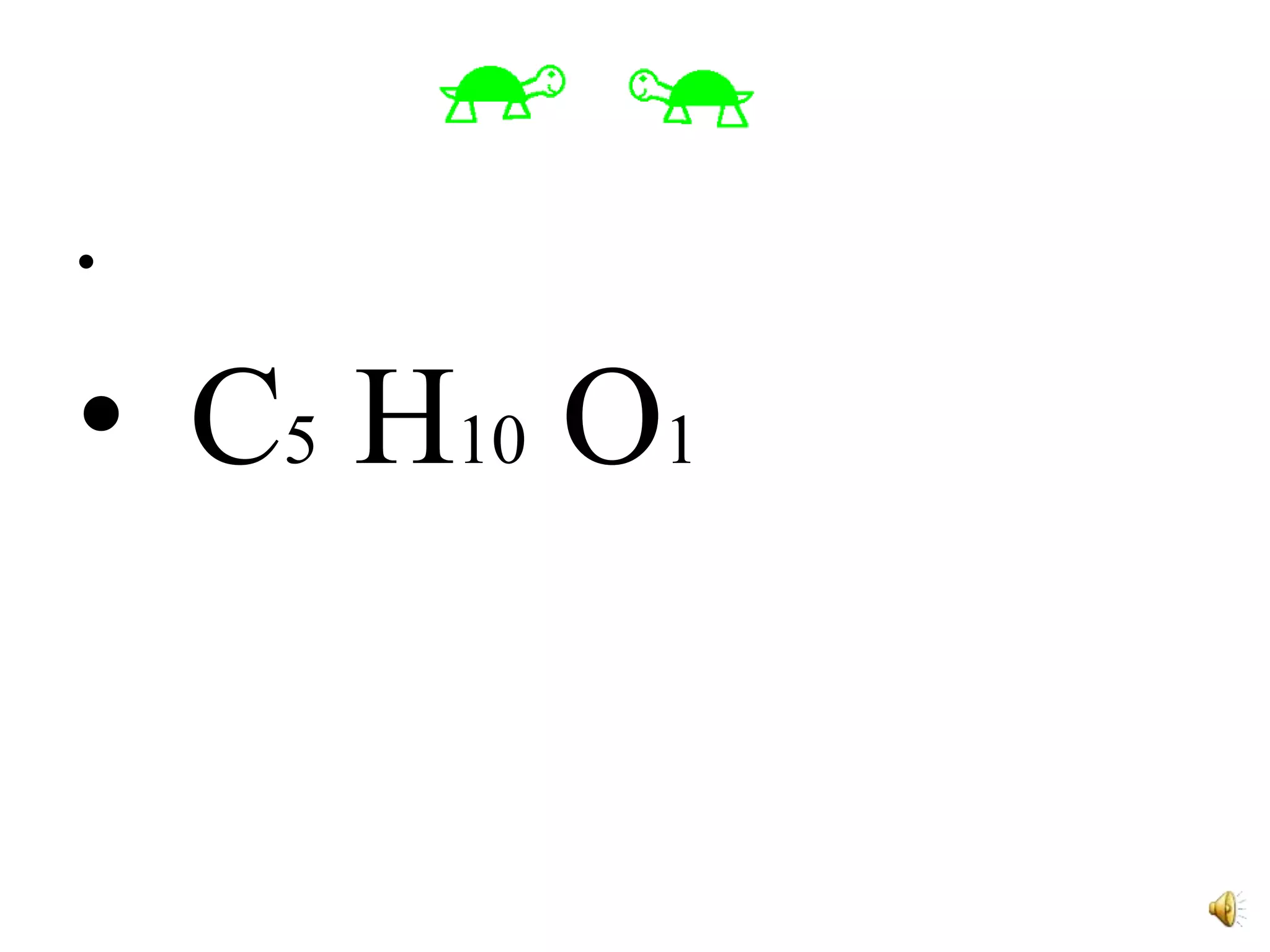
3) The ratios of each element are then calculated by dividing the moles of each element by the smallest value, in this case oxygen, to determine the simplest formula of C5H10O1.