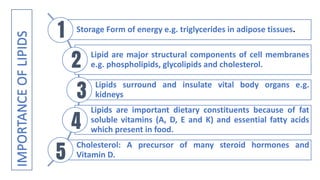
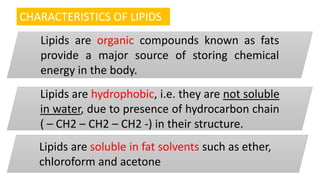
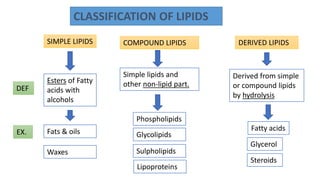
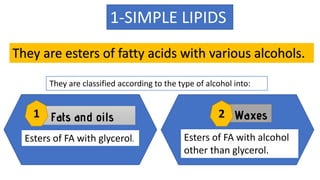
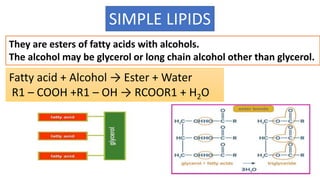
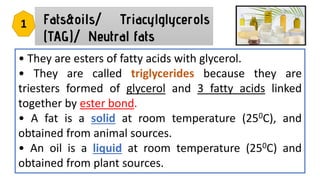
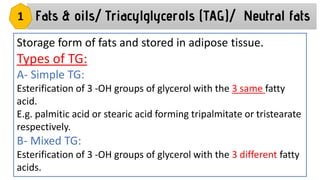
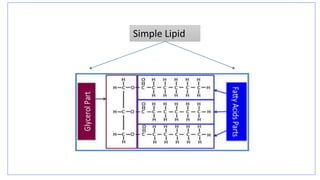
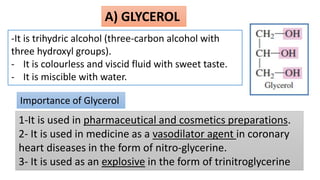
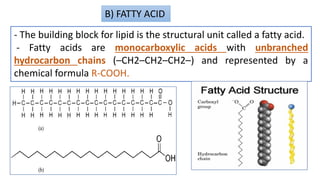
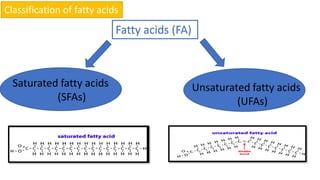
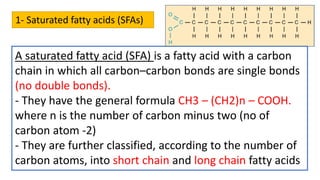
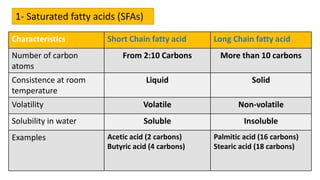
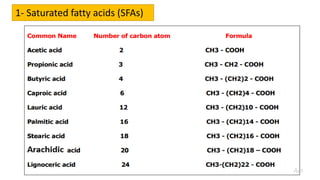
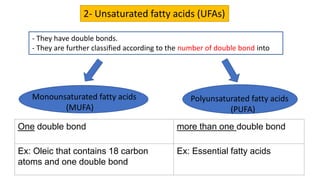
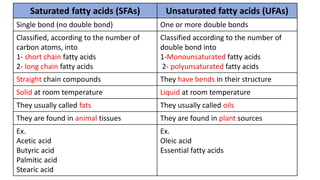
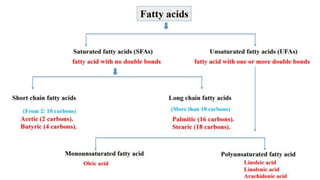
This document discusses lipids and their classification. It defines lipids as organic compounds that provide stored energy and are hydrophobic. Lipids are classified as simple, compound, or derived. Simple lipids include fats, oils, and waxes. Fats and oils are triglycerides consisting of glycerol bonded to three fatty acids. They provide long-term energy storage. Waxes are fatty acid esters of long-chain alcohols. The document also describes the structures of fatty acids and glycerol, which are components of lipids. Saturated fatty acids have only single bonds while unsaturated fatty acids contain one or more double bonds. Some unsaturated fatty acids are essential to obtain from diet.