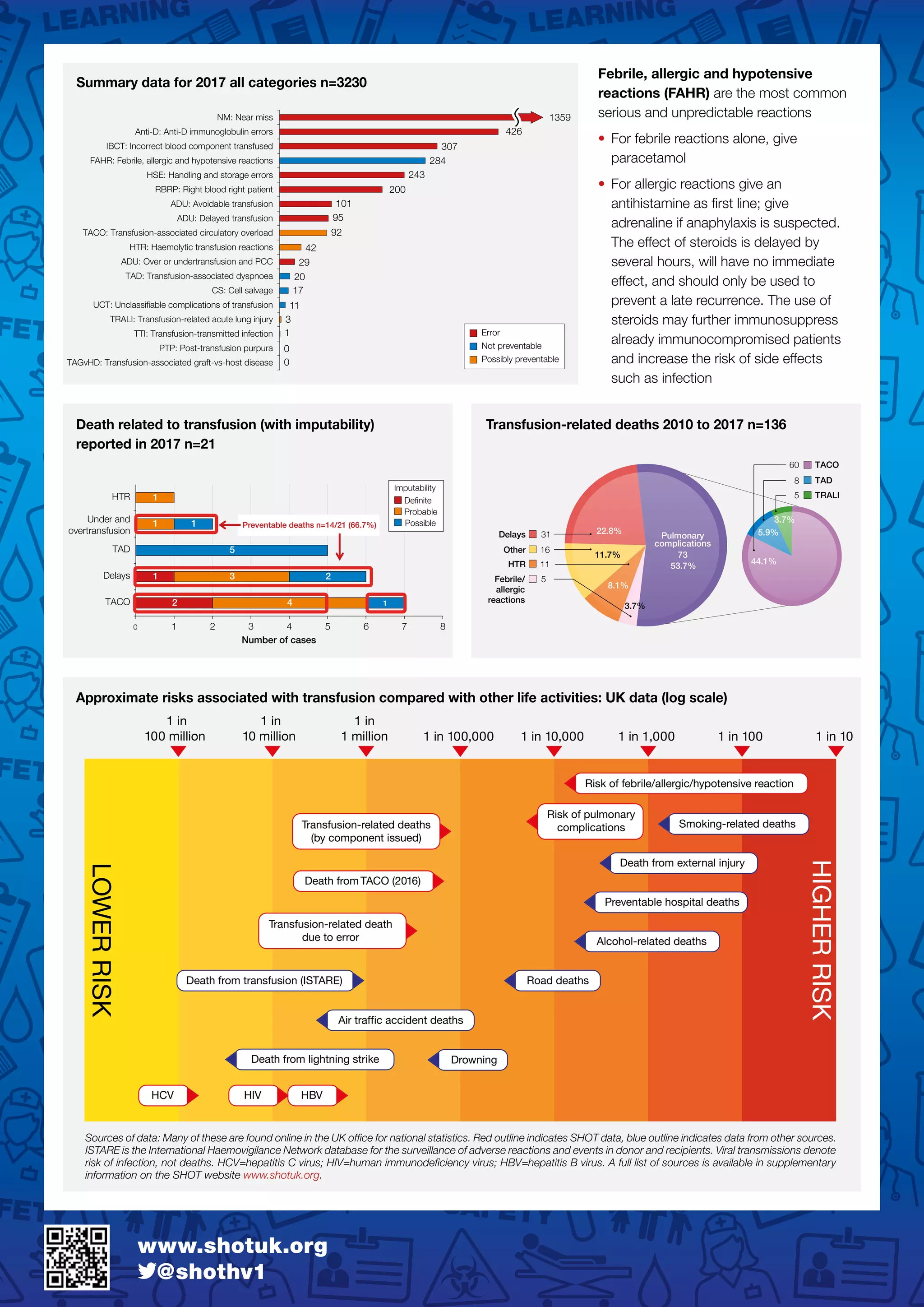
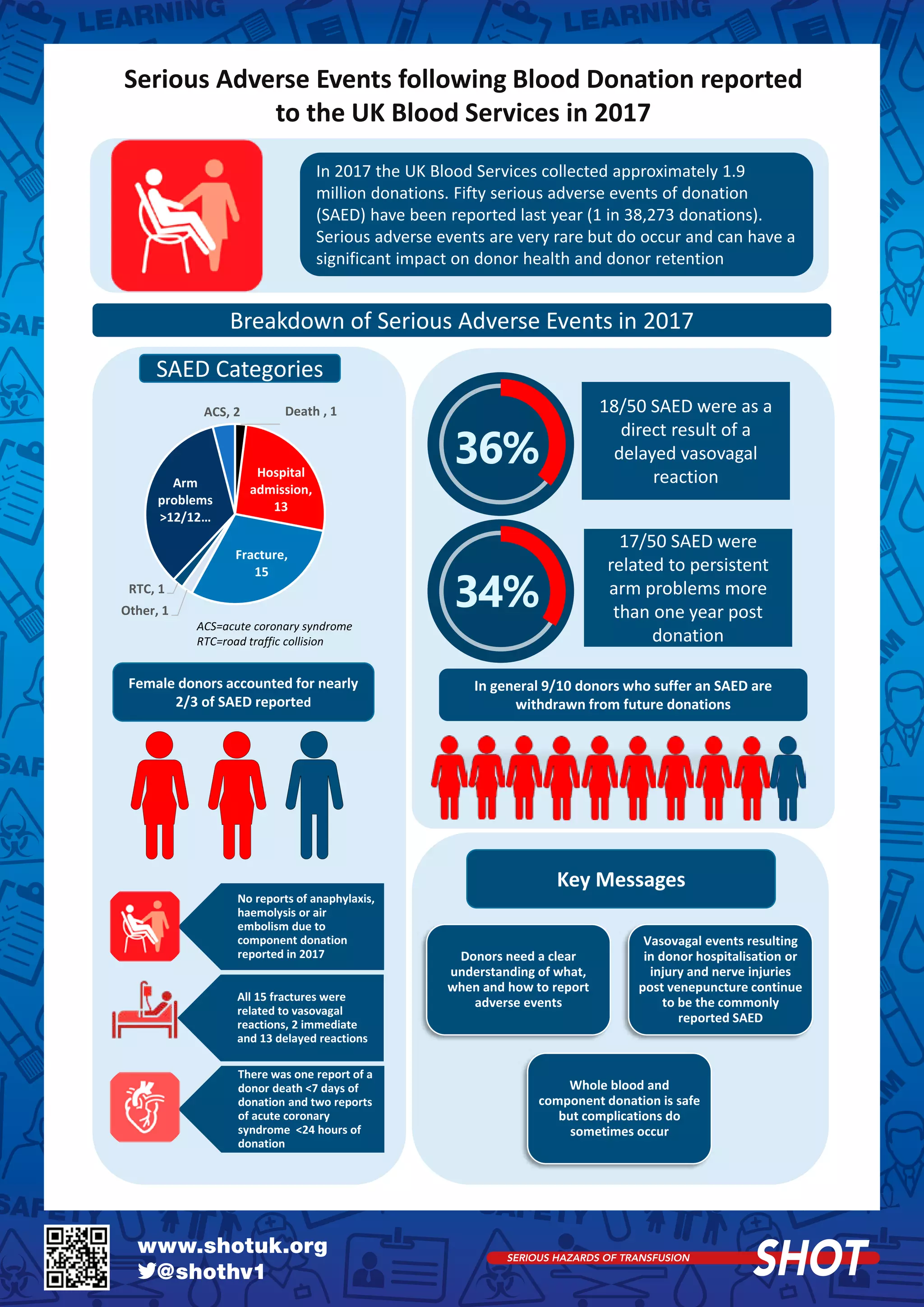
1) The annual SHOT report summarizes transfusion-related adverse events in the UK in 2017. There were a total of 3,230 reports, with 85.5% considered errors. 21 deaths occurred, with 14 deemed preventable.
2) Key issues included failures in patient identification, communication, and following guidelines. Inadequate staffing, training, and supervision increased error risk. Electronic systems can help reduce errors if fully utilized.
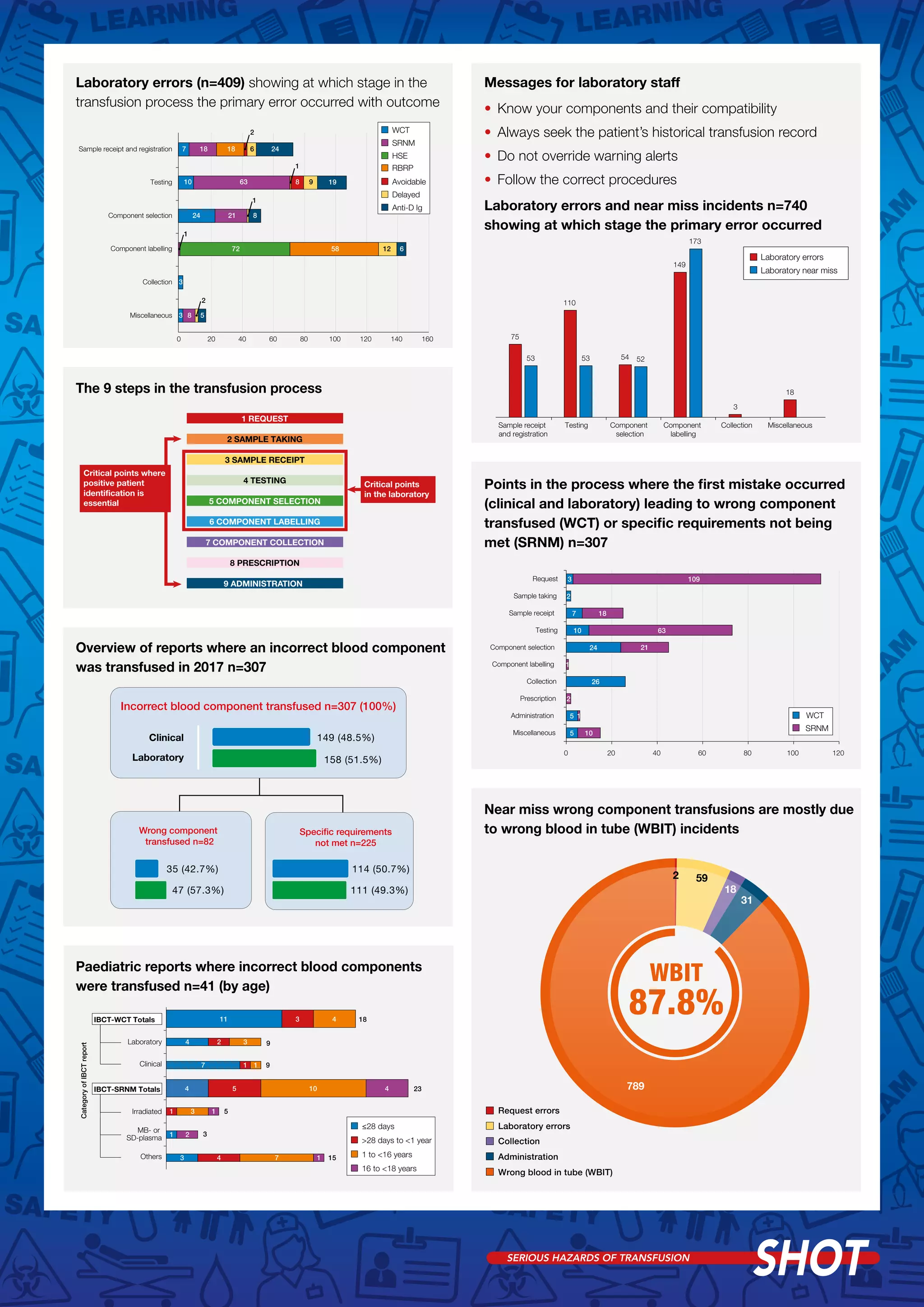
3) 1 ABO-incompatible red blood cell transfusion occurred due to administration error, along with 4 incompatible FFP and 2 platelets. Improved training in blood group principles was recommended.