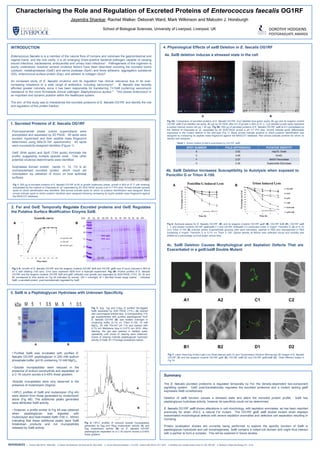
The excreted proteome of Enterococcus faecalis OG1RF is temporally regulated by the Fsr two-component signalling system. GelE post-translationally regulates excreted proteins and a gelE mutant constitutively expresses SalB. Deletion of salB causes stress and alters the excreted protein profile. SalB has peptidoglycan hydrolase activity but its specificity could not be determined. A salB mutant shows cell morphology and septation defects that are exacerbated in a gelE/salB double mutant, with severe septation anomalies and defective cell separation.