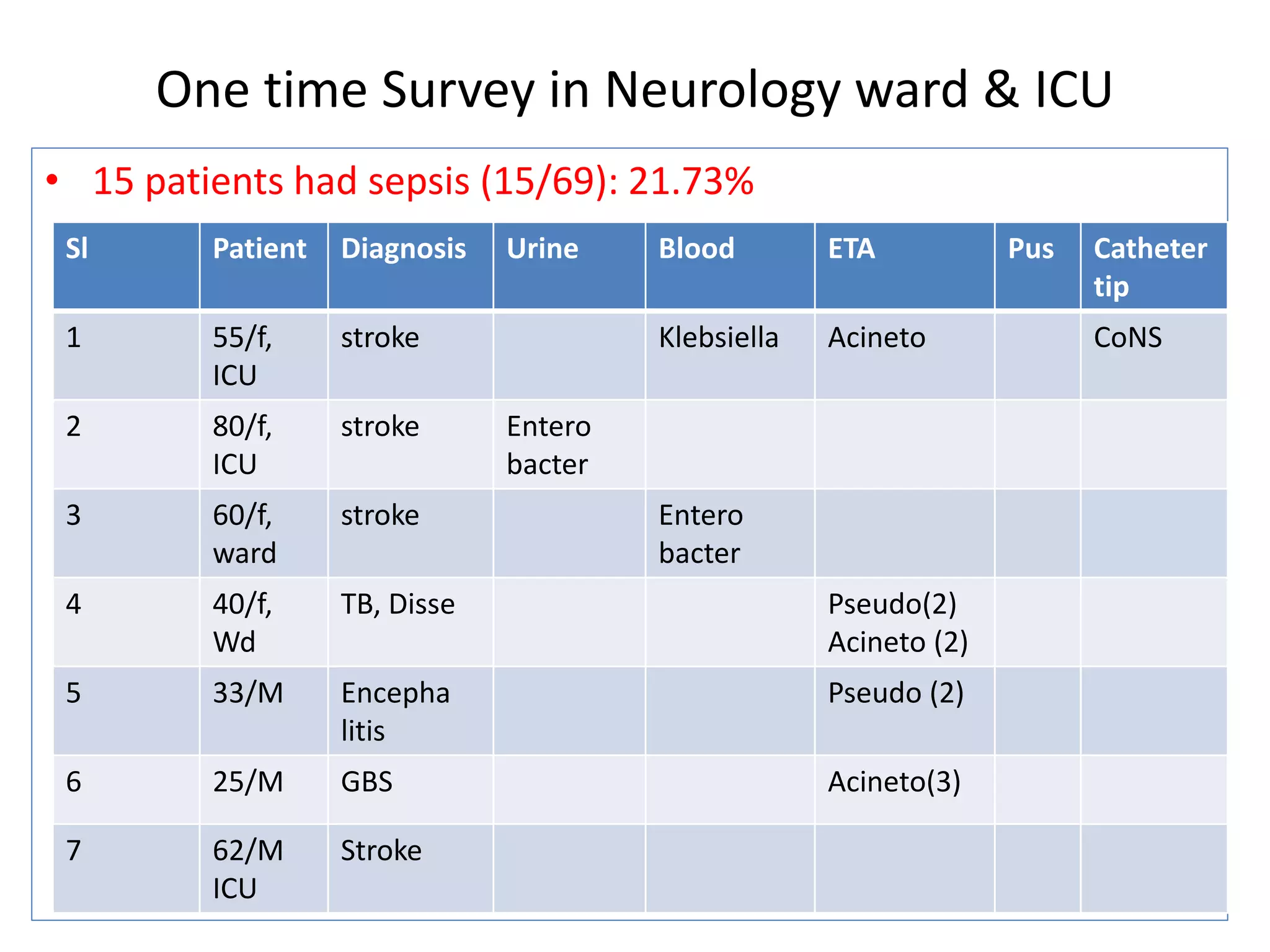
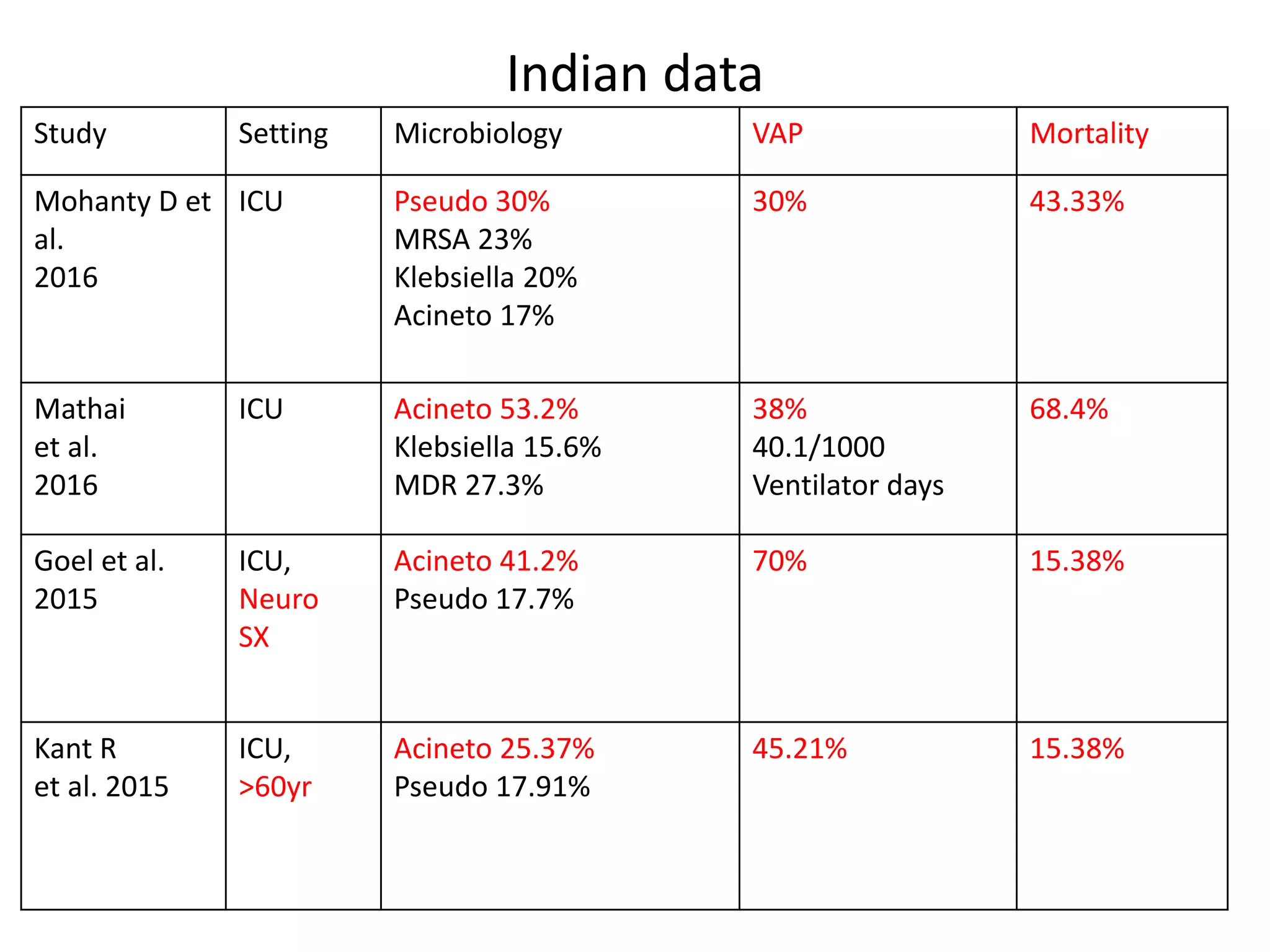
1) A one-time survey in a neurology ward and ICU found that 15 out of 69 patients (21.73%) had sepsis. Common organisms found included Klebsiella, Enterobacter, Pseudomonas, Acinetobacter, and E. coli.
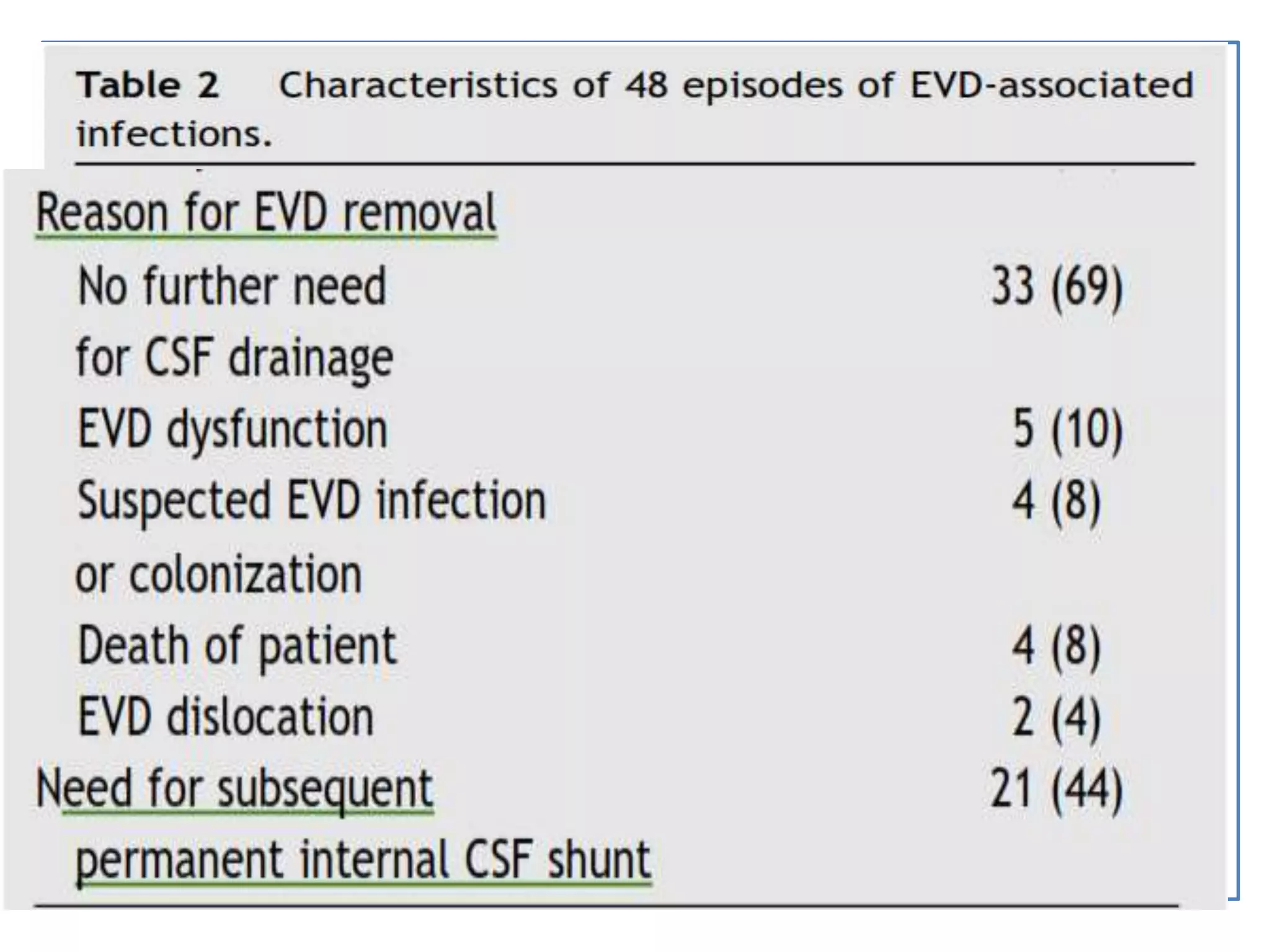
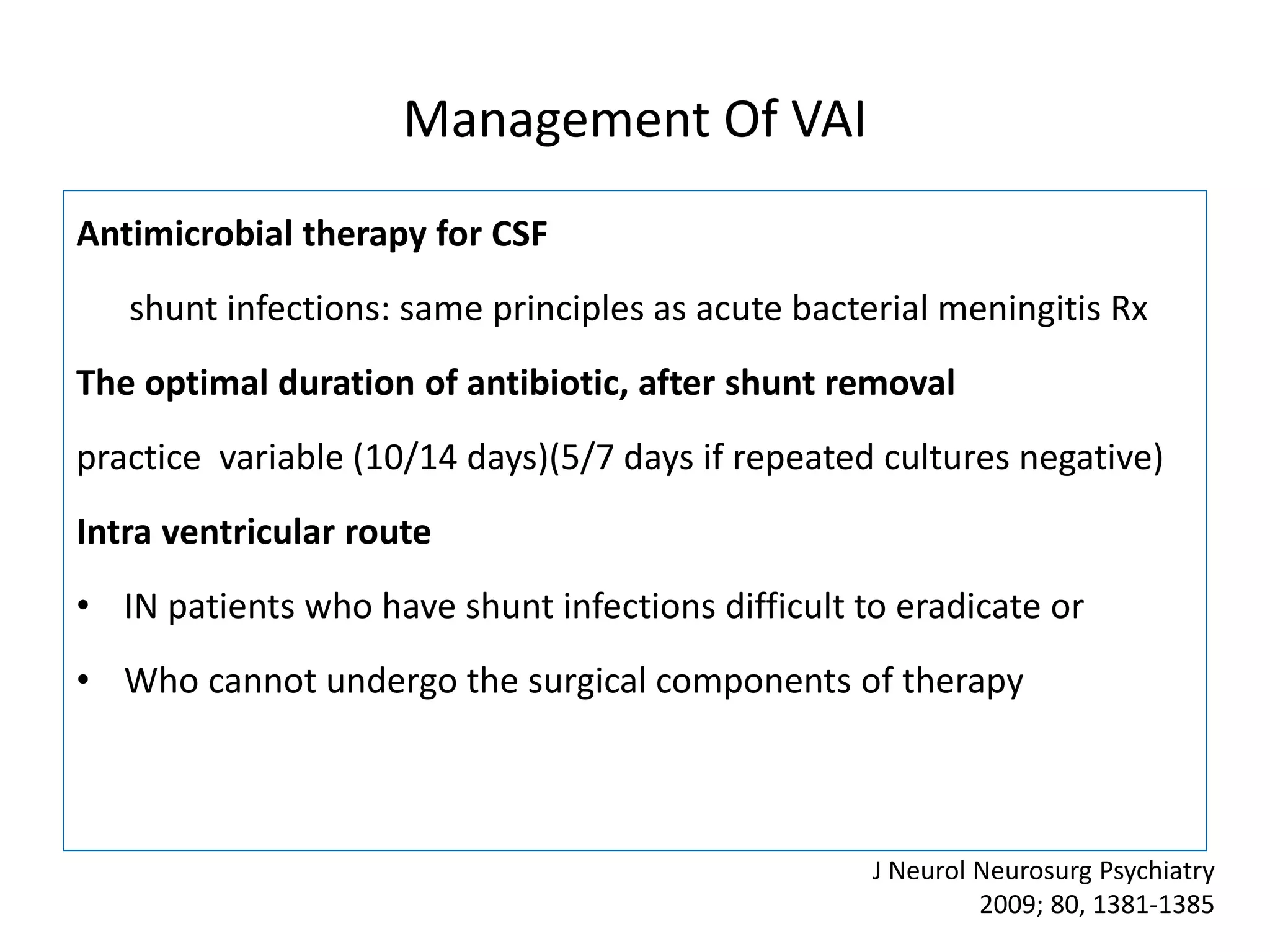
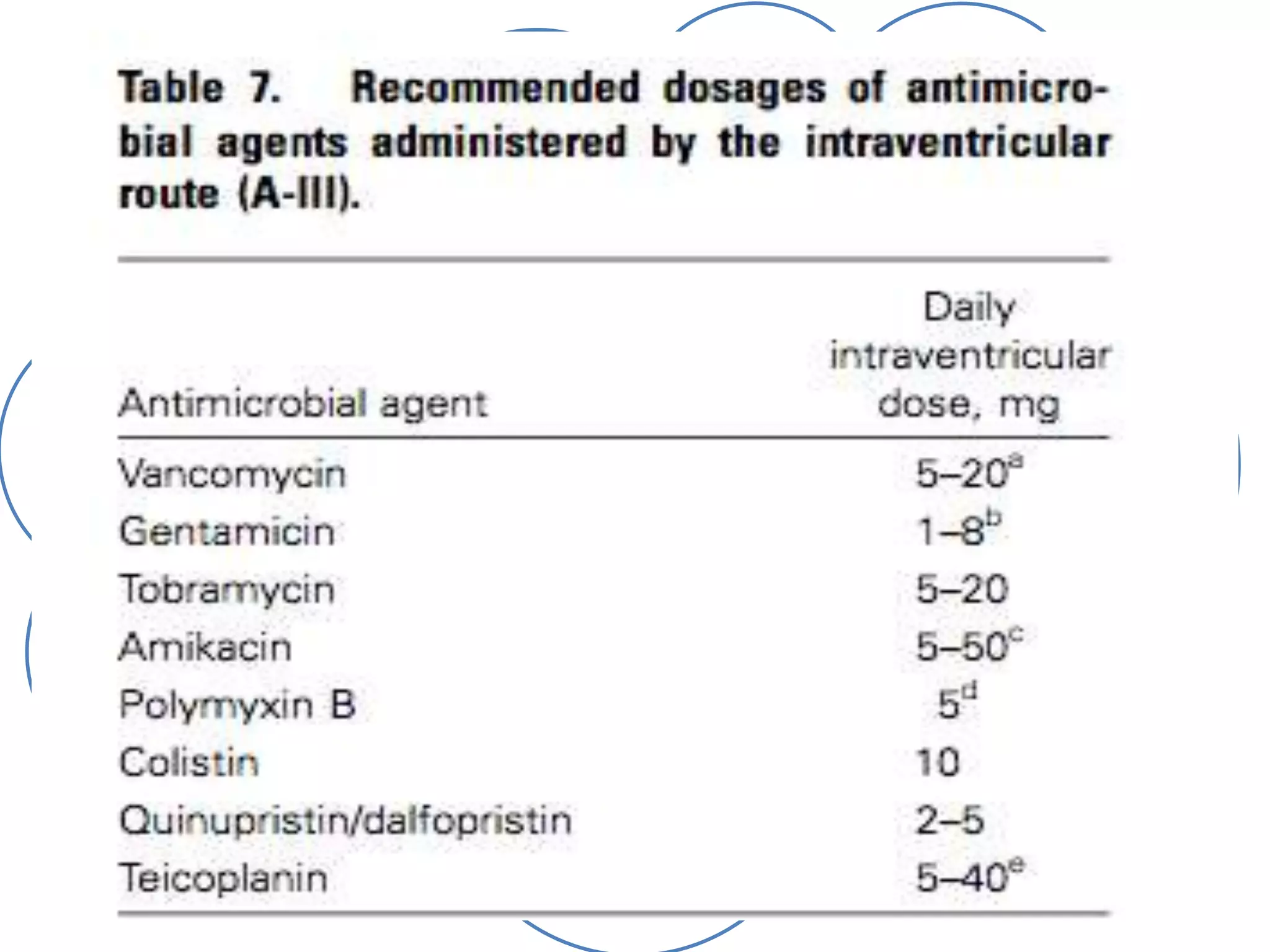
2) Guidelines for treating ventriculostomy-associated infections recommend intravenous and intraventricular antibiotics such as vancomycin. Combined treatment may improve outcomes over intravenous antibiotics alone.
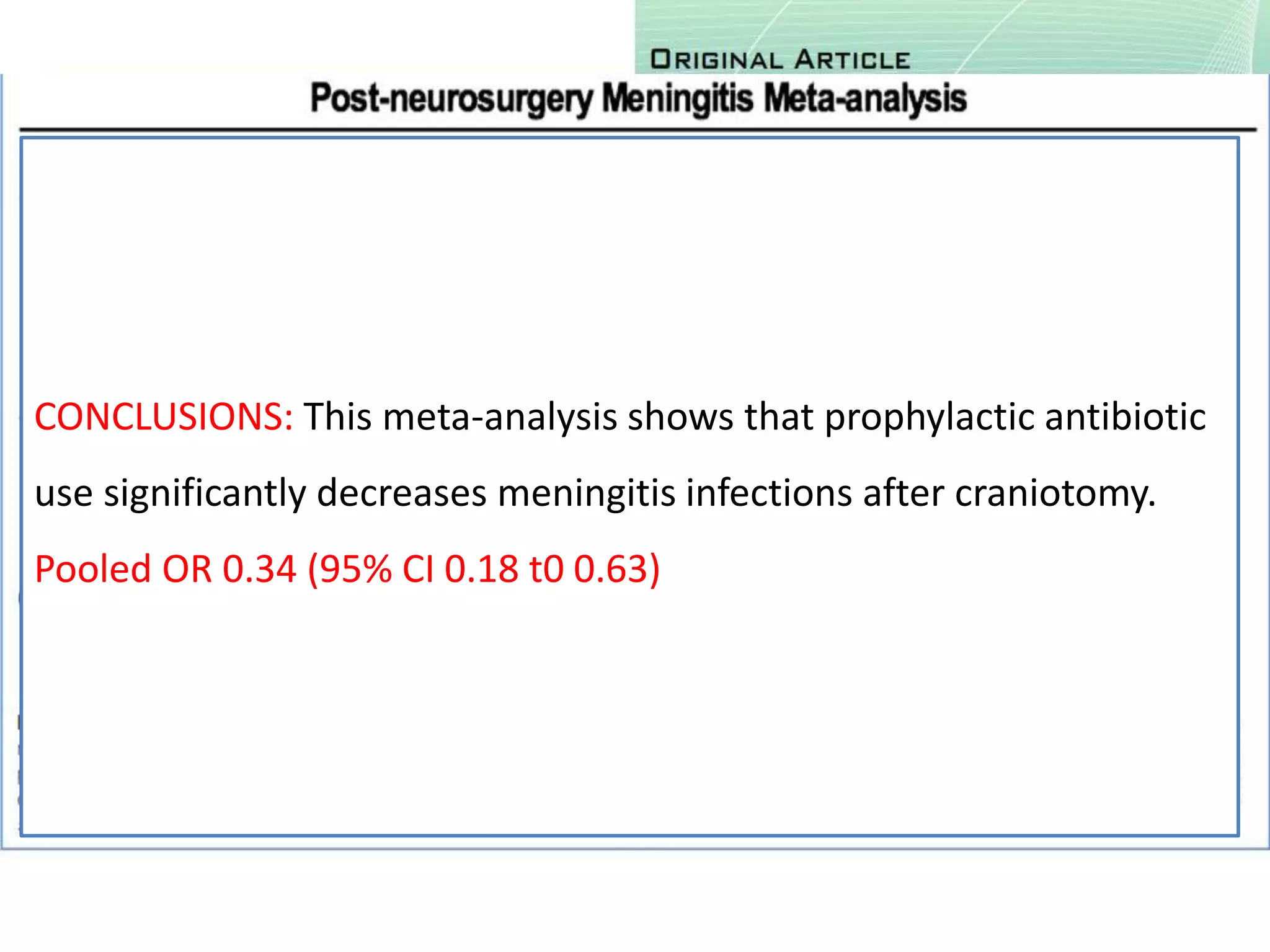
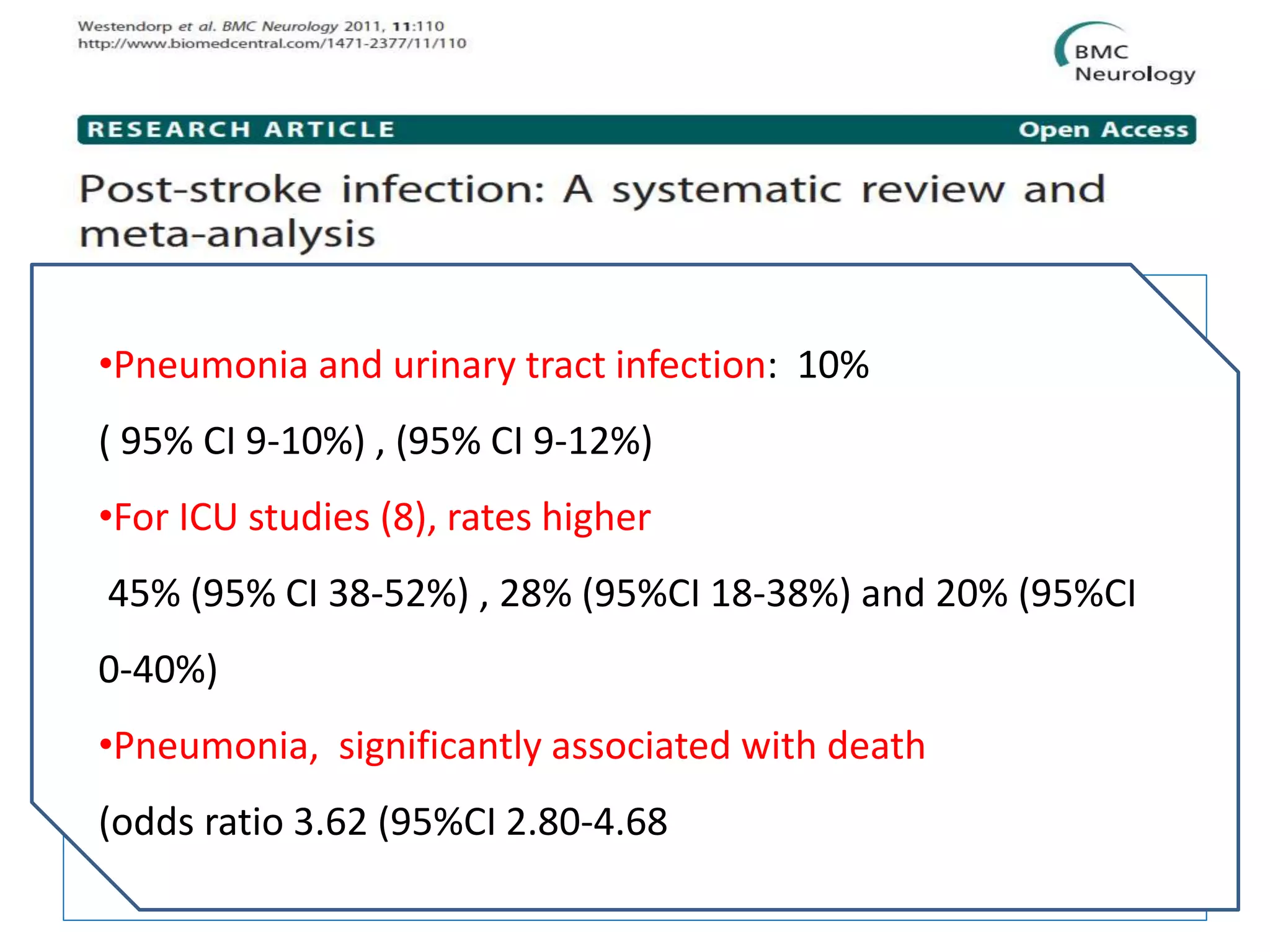
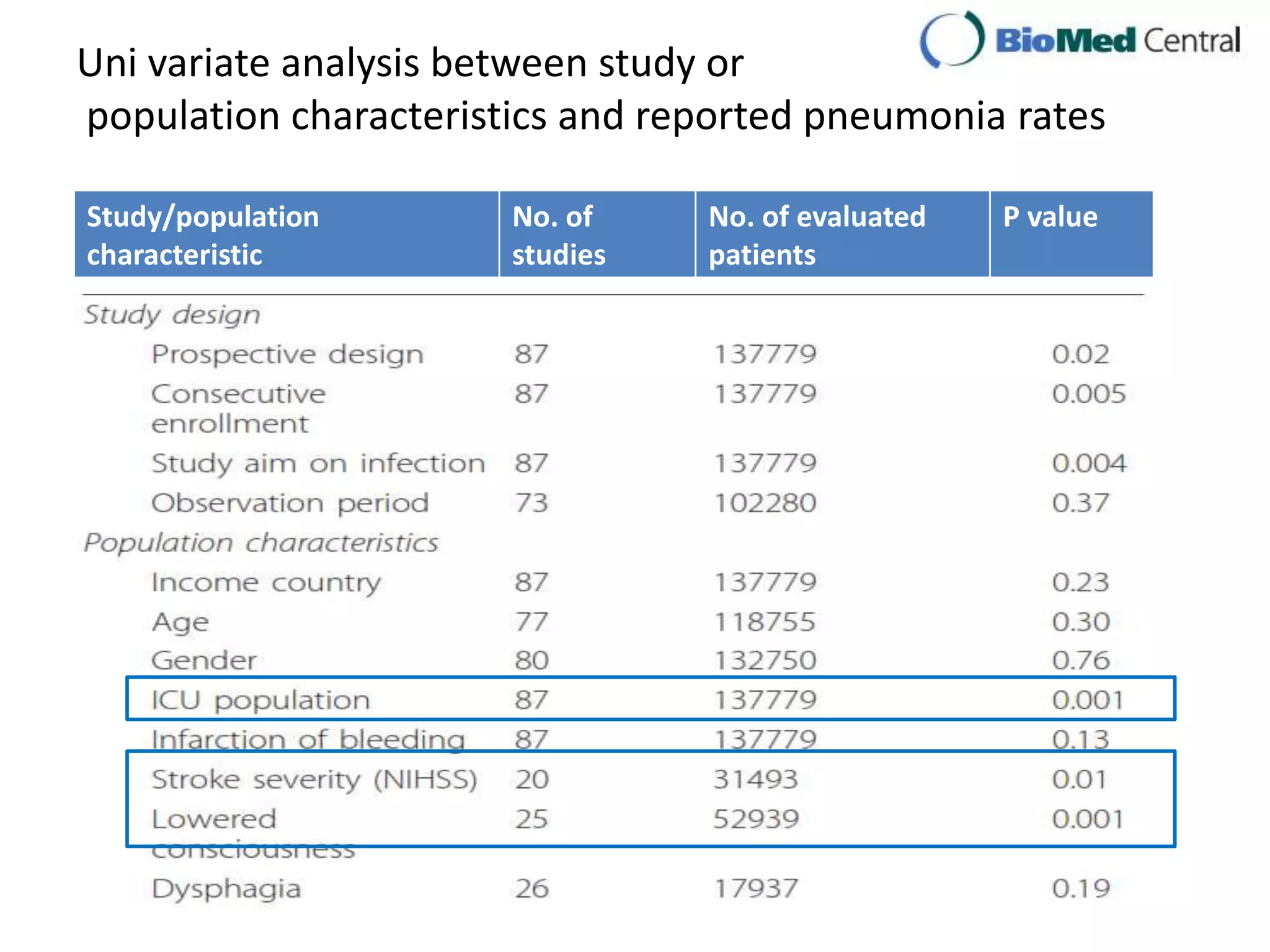
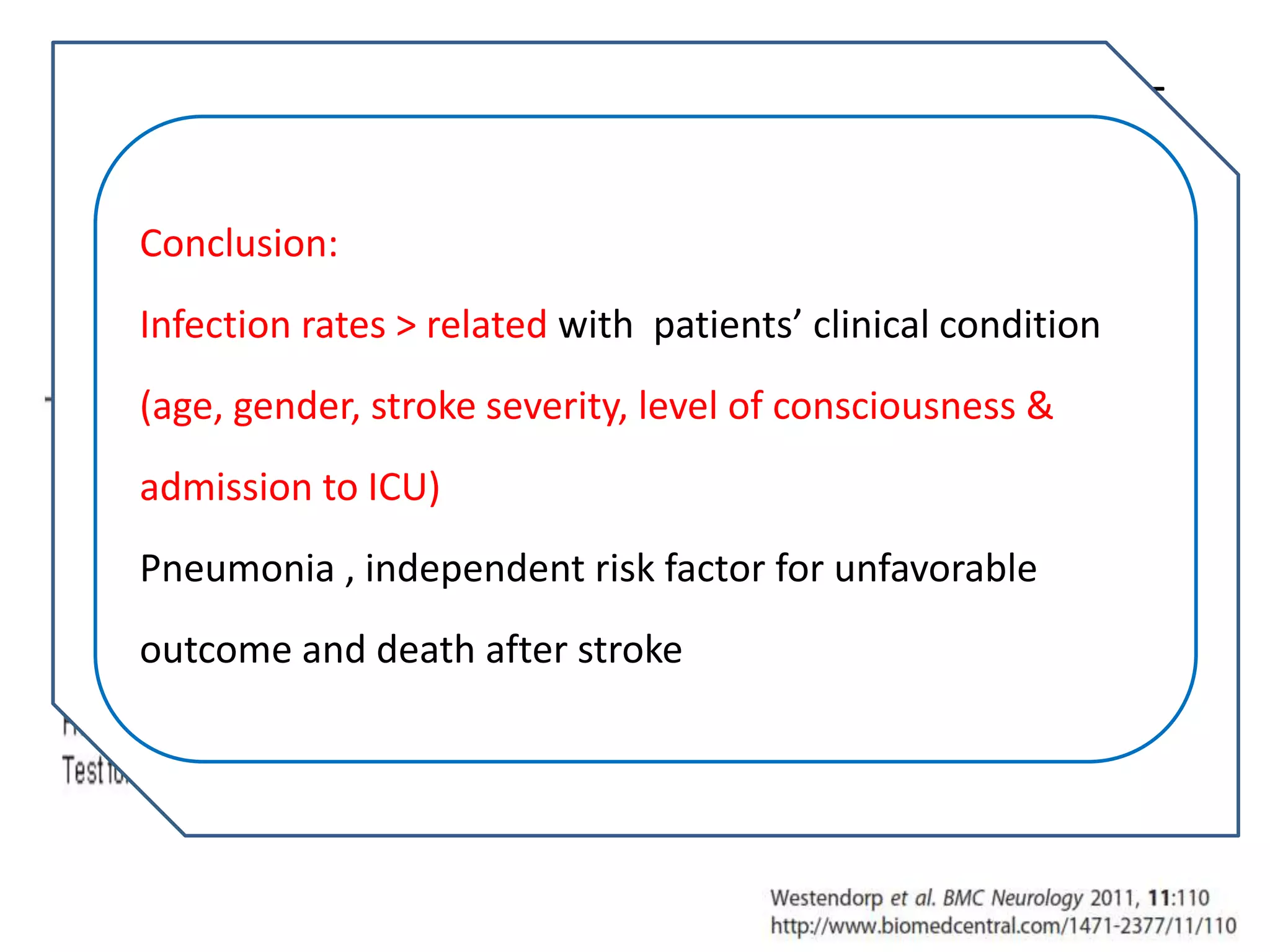
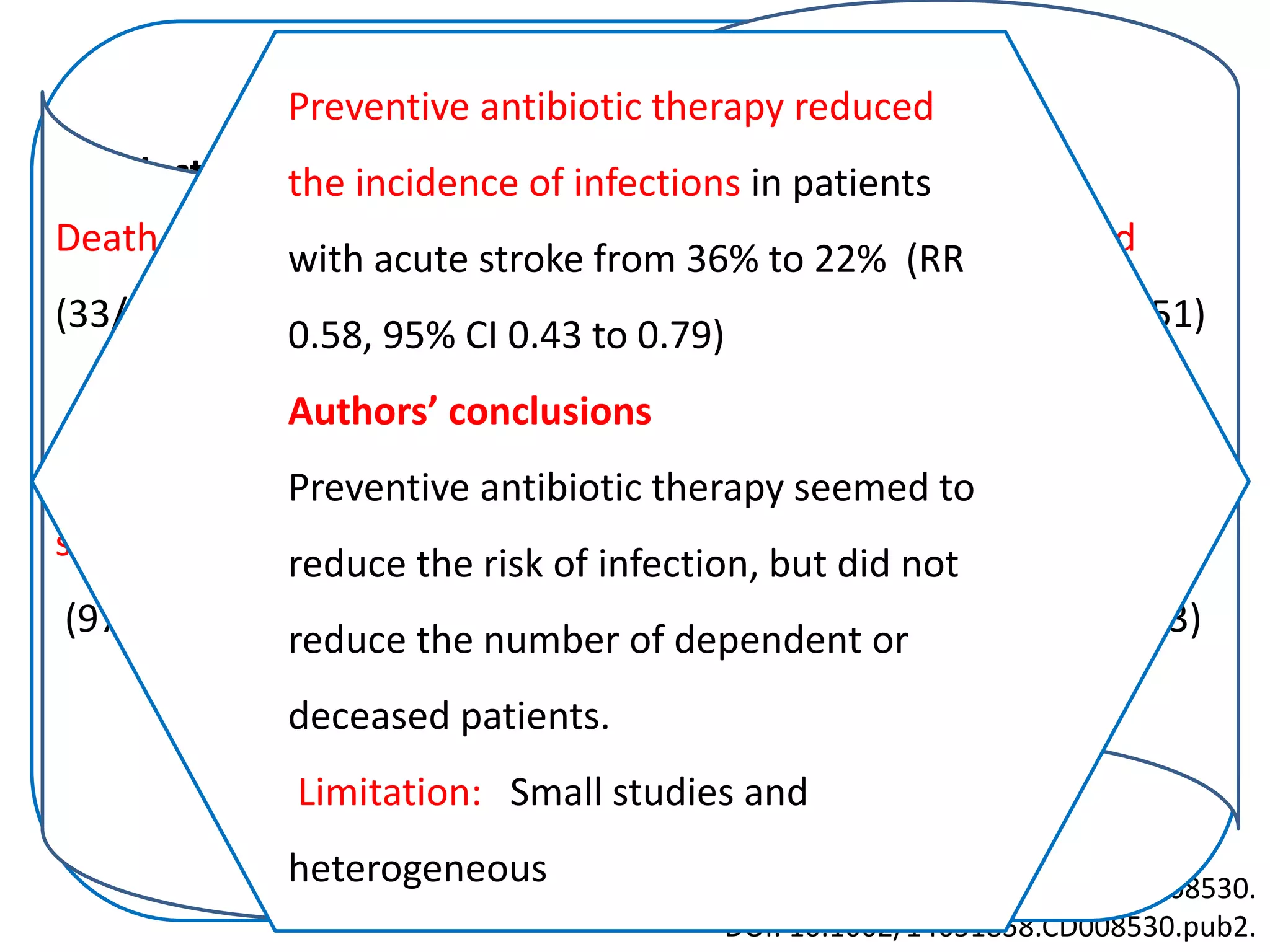
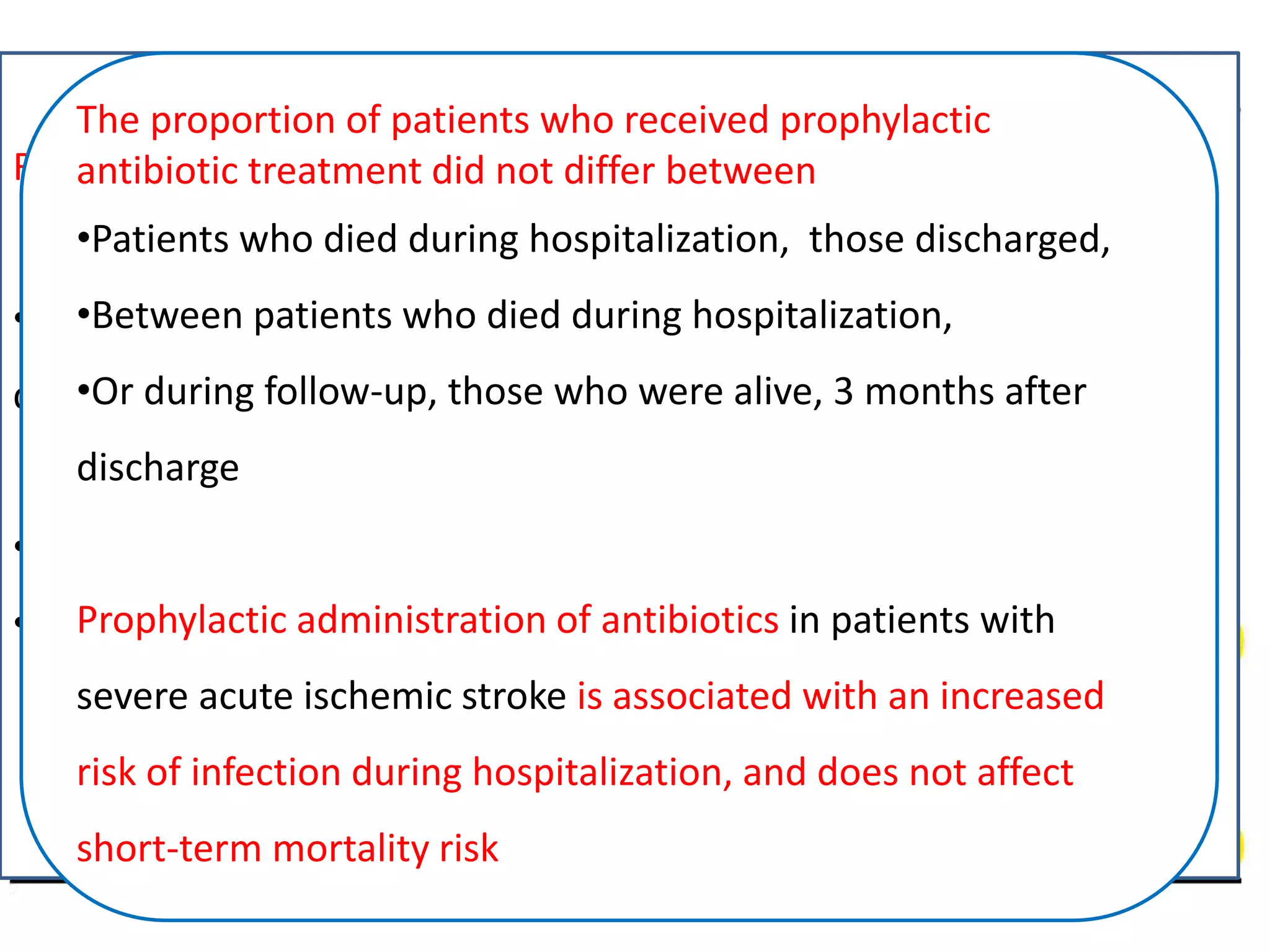
3) Post-stroke infections are common, with reported rates around 30%. Pneumonia is the most frequent type of infection and is associated with increased mortality. Preventive antibiotics may reduce infection rates but not affect mortality.






















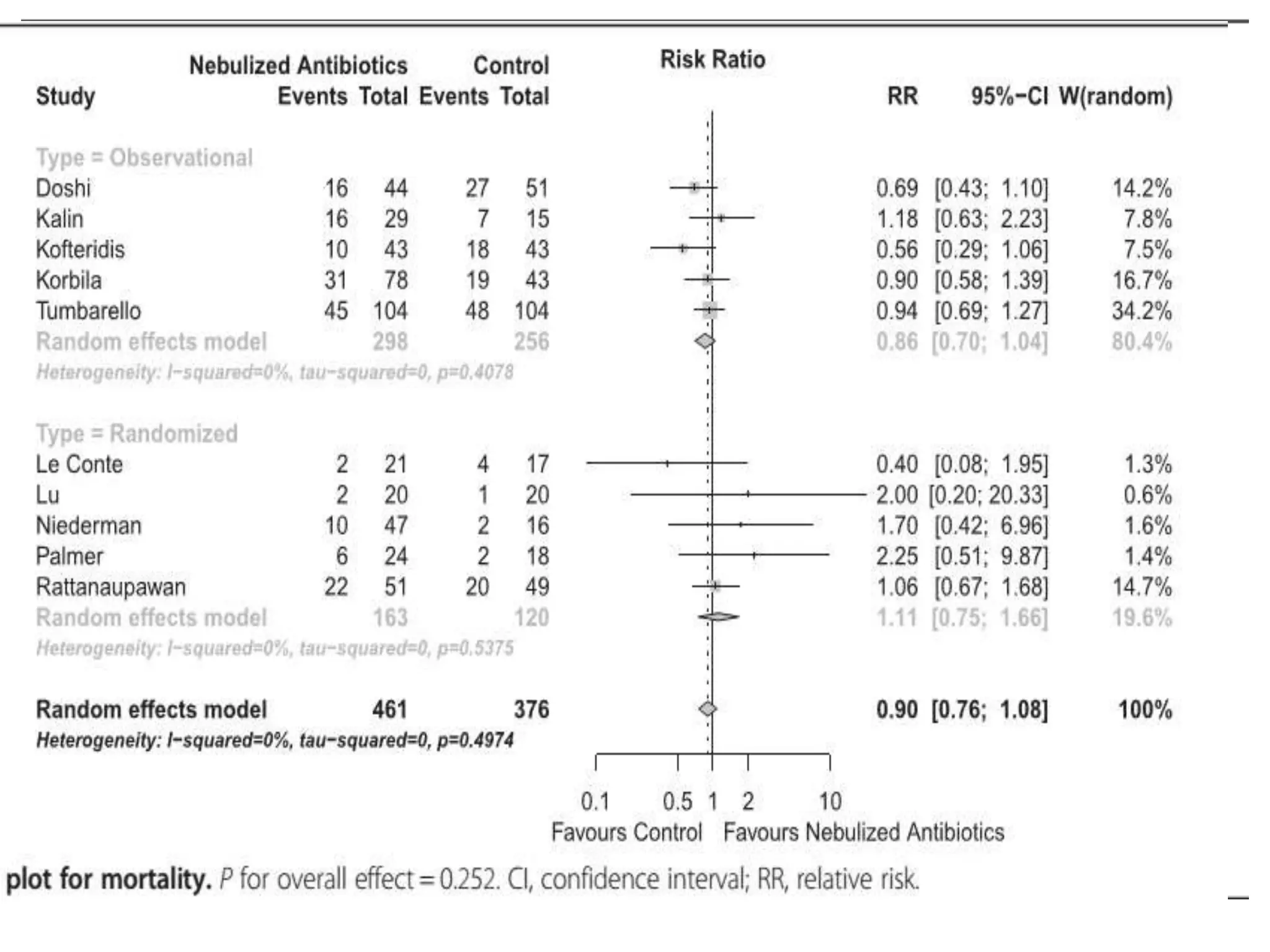











![Total Neurology (AIIMS) Healthcare associated
infection (HCAI) data for July 2016
Pathogens
MRSA E.coli Pseudomonas Acinetobacter Enterobacter Total
1, Blood 1, Urine 1, Blood 2, Chest 1, Blood
1, urine
2, chest
9
MRSA E.coli Pseudomonas Acinetobacter Enterobacter
Linezolid (100%)
Vancomycin
(100%)
Netilmycin (100%)
Amikacin
(100%)
Netimycin
(100%)
Amikacin
(100%)
Pip/Taz(100%)
Colistin(100%)
Cef/Sulb
(100%)
Colistin (100%)
Colistin 75%
Cef/sulb 25%
Pip/Taz 25%
Amikacin 25%
Total
admissions
CSF Blood Chest UTI Total
208 0 2 5 2 9 [4.32%]
Sensitivity](https://image.slidesharecdn.com/seminarsepsisnew-160913074928/75/Sepsis-and-antibiotic-guidance-in-neurology-wards-52-2048.jpg)
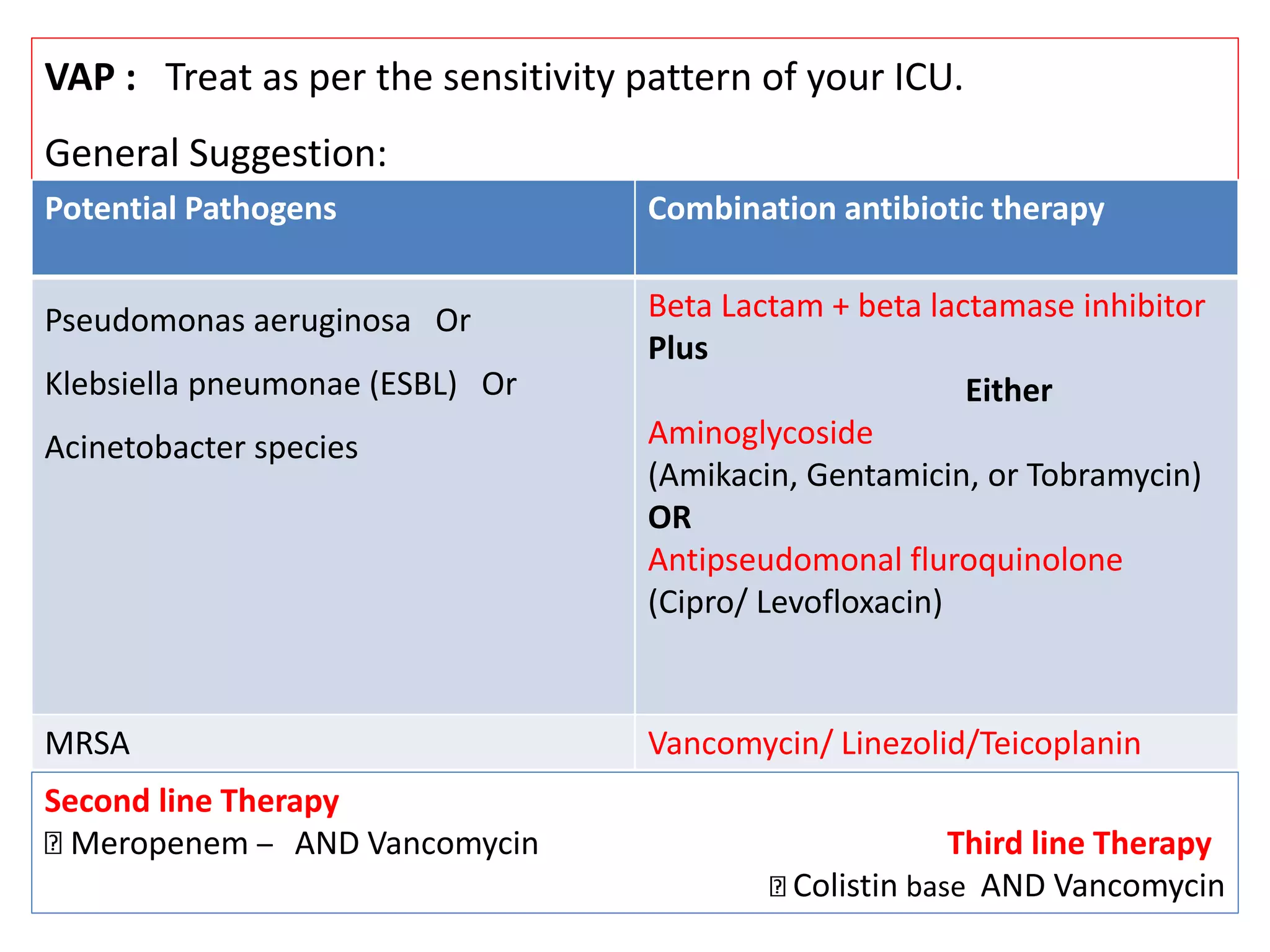
![Total Neurology (AIIMS) Healthcare associated
infection (HCAI) data for June 2016
Pathogens
E.coli Pseudomonas Acinetobacter Enterococcus Total
1, Urine 1, Chest 1, Chest 1, Blood 1
E.coli Pseudomonas Acinetobacter Enterococcus
Amikacin
Cefotaxime
Ceftazidime
Cef+sulbactum
Nitrofurantoin
Netimycin
Pip+ Tazobactum
Colistin Cef/Sulb
(100%)
Colistin (100%)
Cefotaxim,
Erythromycin,
Linezolid,
Penicillin,
Teicoplanin,
Vancomycin
Total
admissions
CSF Blood Chest UTI Total
213 0 1 2 1 4 [1.87%]
Sensitivity](https://image.slidesharecdn.com/seminarsepsisnew-160913074928/75/Sepsis-and-antibiotic-guidance-in-neurology-wards-53-2048.jpg)