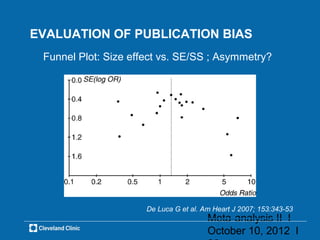
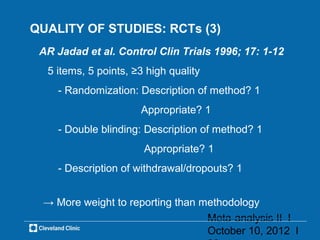
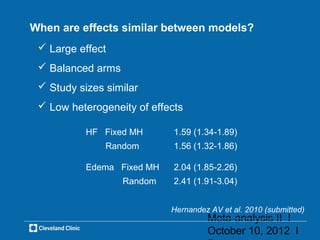
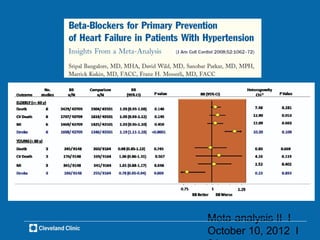
This document outlines key concepts and methodologies related to performing meta-analyses, including types of models (fixed and random effects), methods for combining study effects, and approaches to addressing heterogeneity and publication bias. It discusses the importance of study quality assessment and details reporting guidelines such as PRISMA and MOOSE. The document emphasizes the use of various statistical tests and methods for exploring and evaluating the validity of meta-analytical results.



















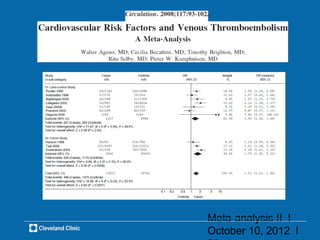
![Use of thiazolidinediones and risk of heart failure and
peripheral edema in patients at high risk of diabetes and
type 2 diabetes:
A systematic review and meta-analysis of placebo-
controlled randomized trials
Follow-up OR (95% CI) RR (95% CI)
≥12 months
MH 1.57 [1.31-1.87] 1.50 [1.28-1.76]
Random 1.67 [1.16-2.40] 1.66 [1.10-2.50]
<12 months
MH 2.71 [0.94-7.79] 2.68 [0.93-7.67]
Random 2.56 [0.88-7.44] 2.52 [0.88-7.25]
Hernandez AV et al. 2010 (Submitted)
Meta-analysis II l
October 10, 2012 l](https://image.slidesharecdn.com/secondpartmaoct202010-13498428603865-phpapp02-121009232240-phpapp02/85/Second-Part-MA-Oct202010-23-320.jpg)