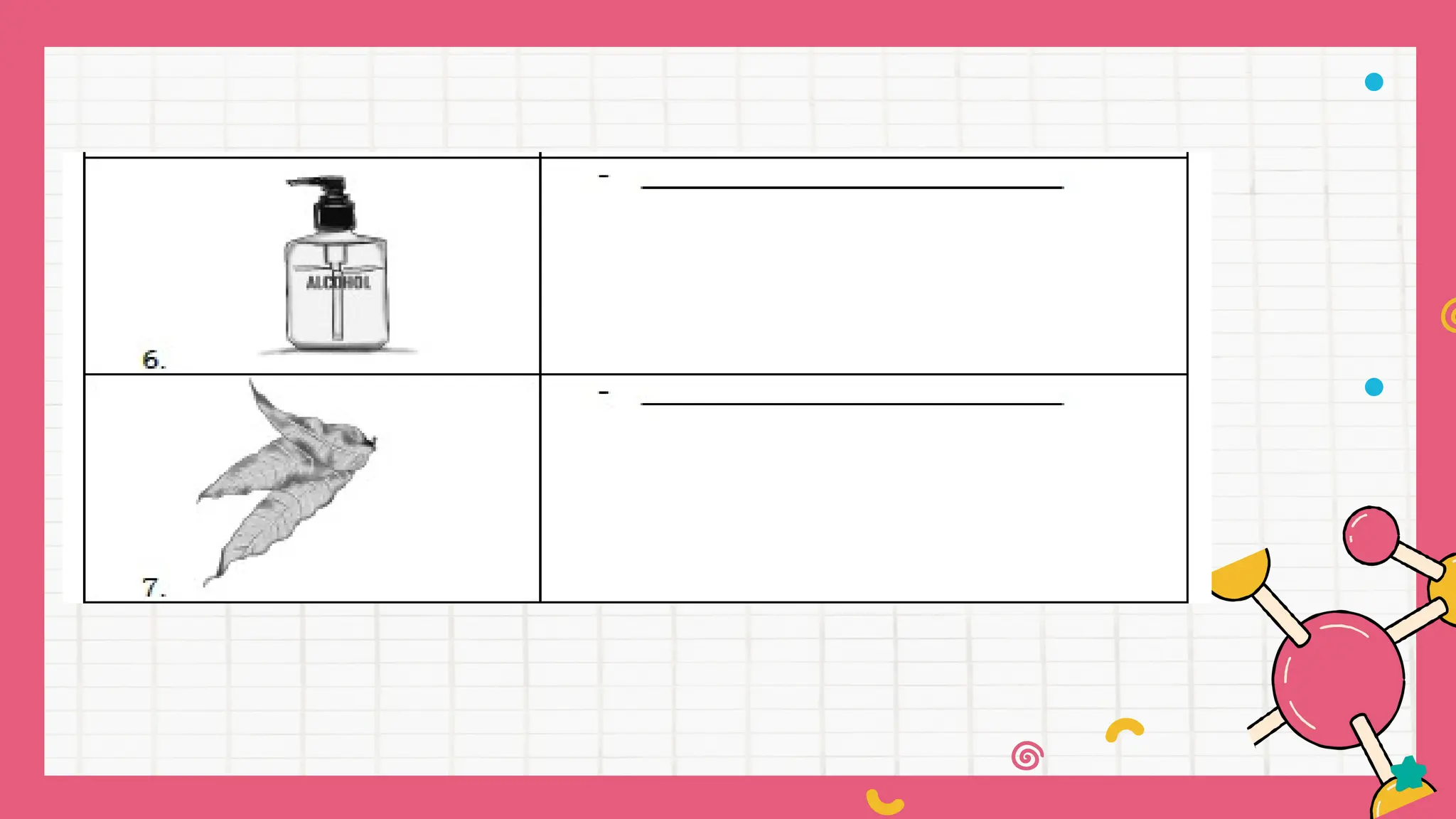
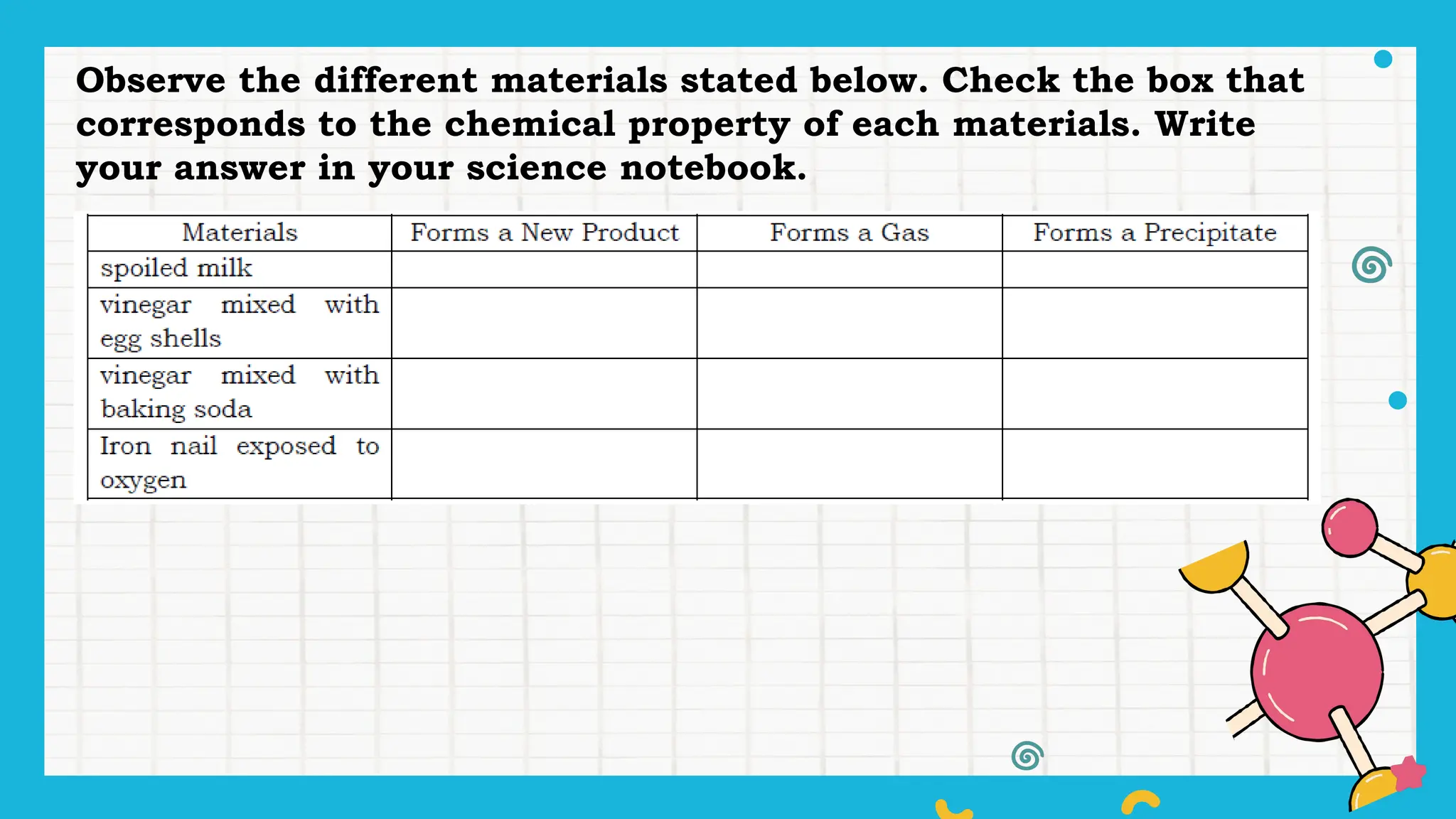
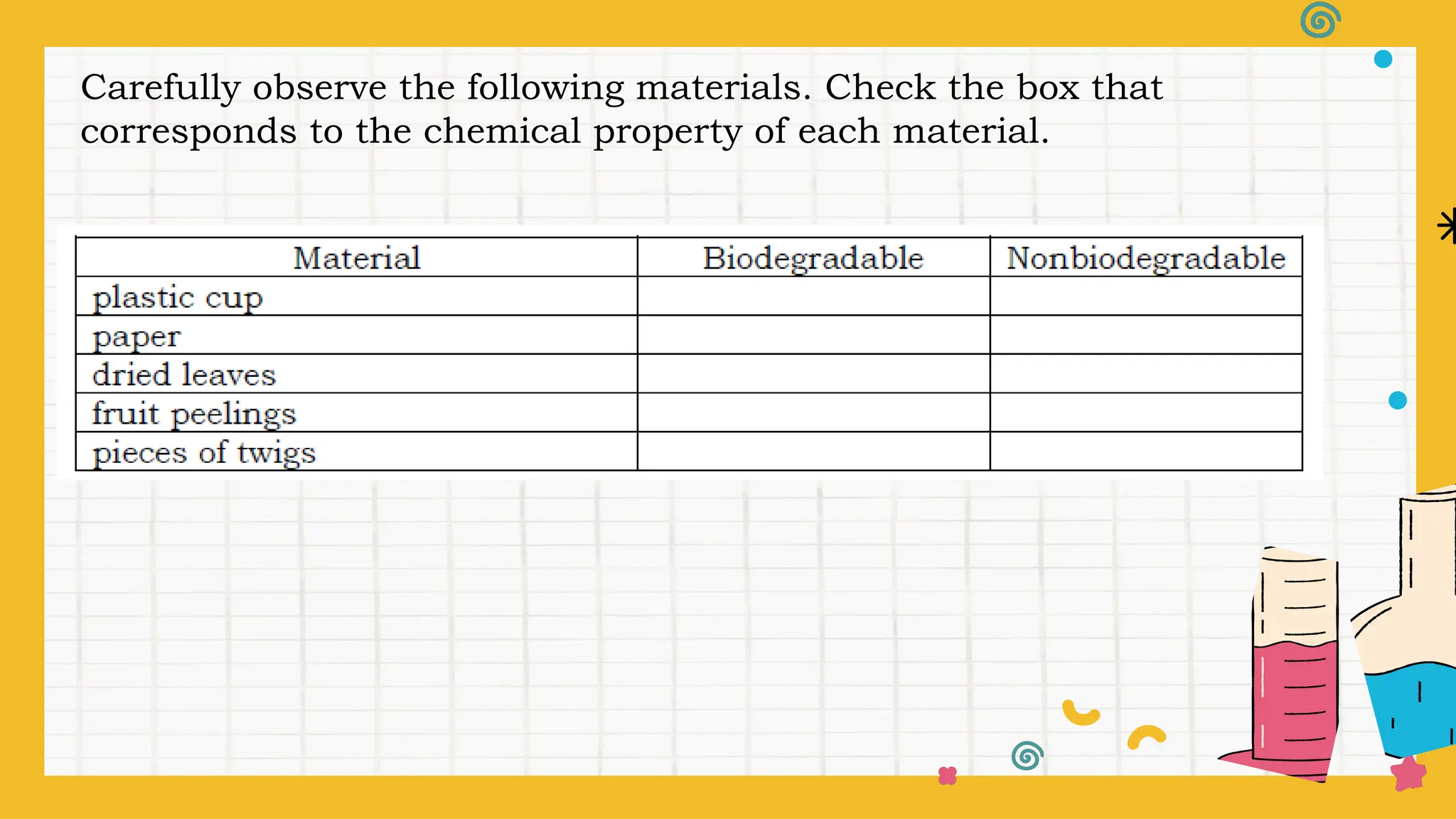
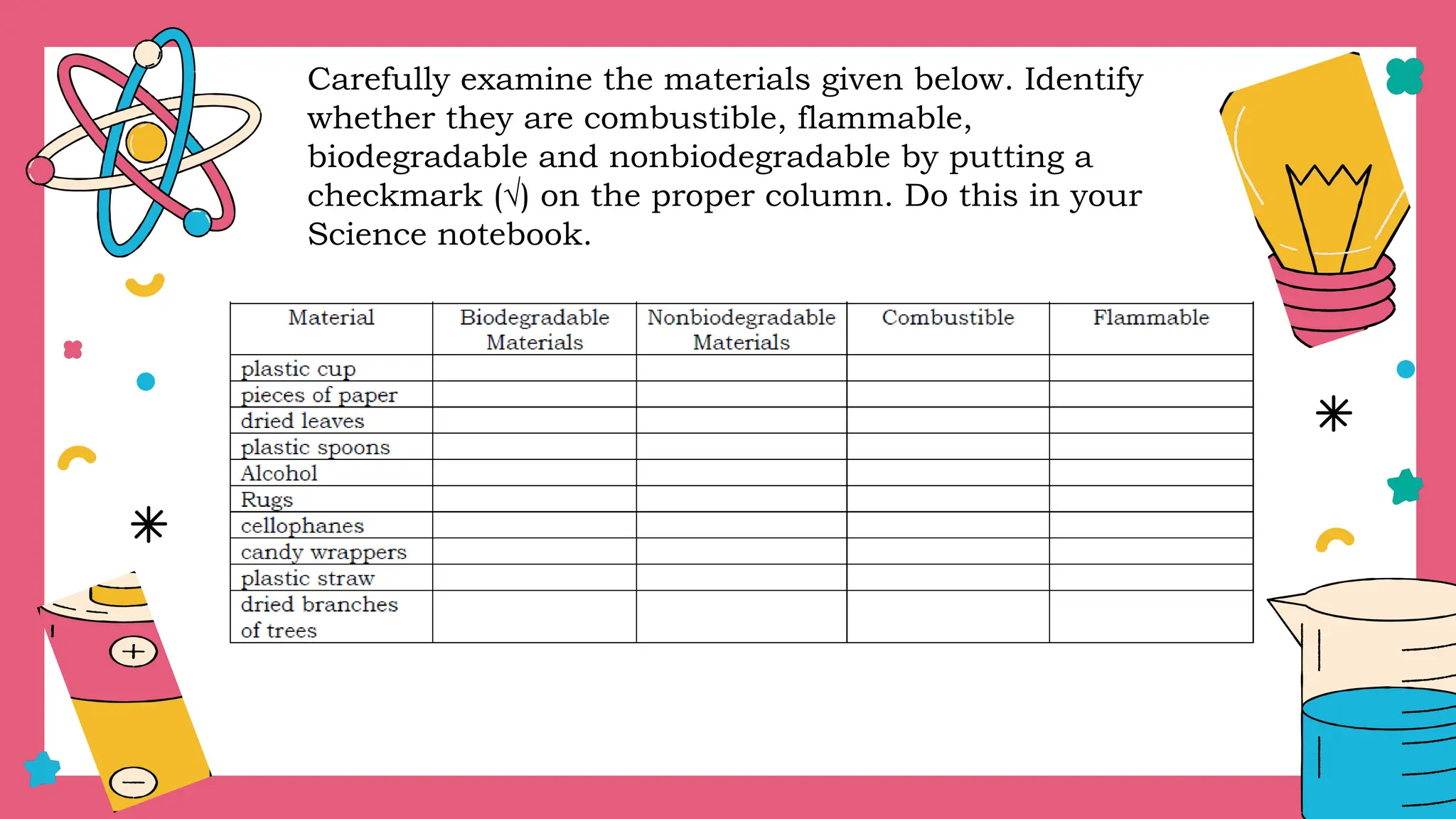
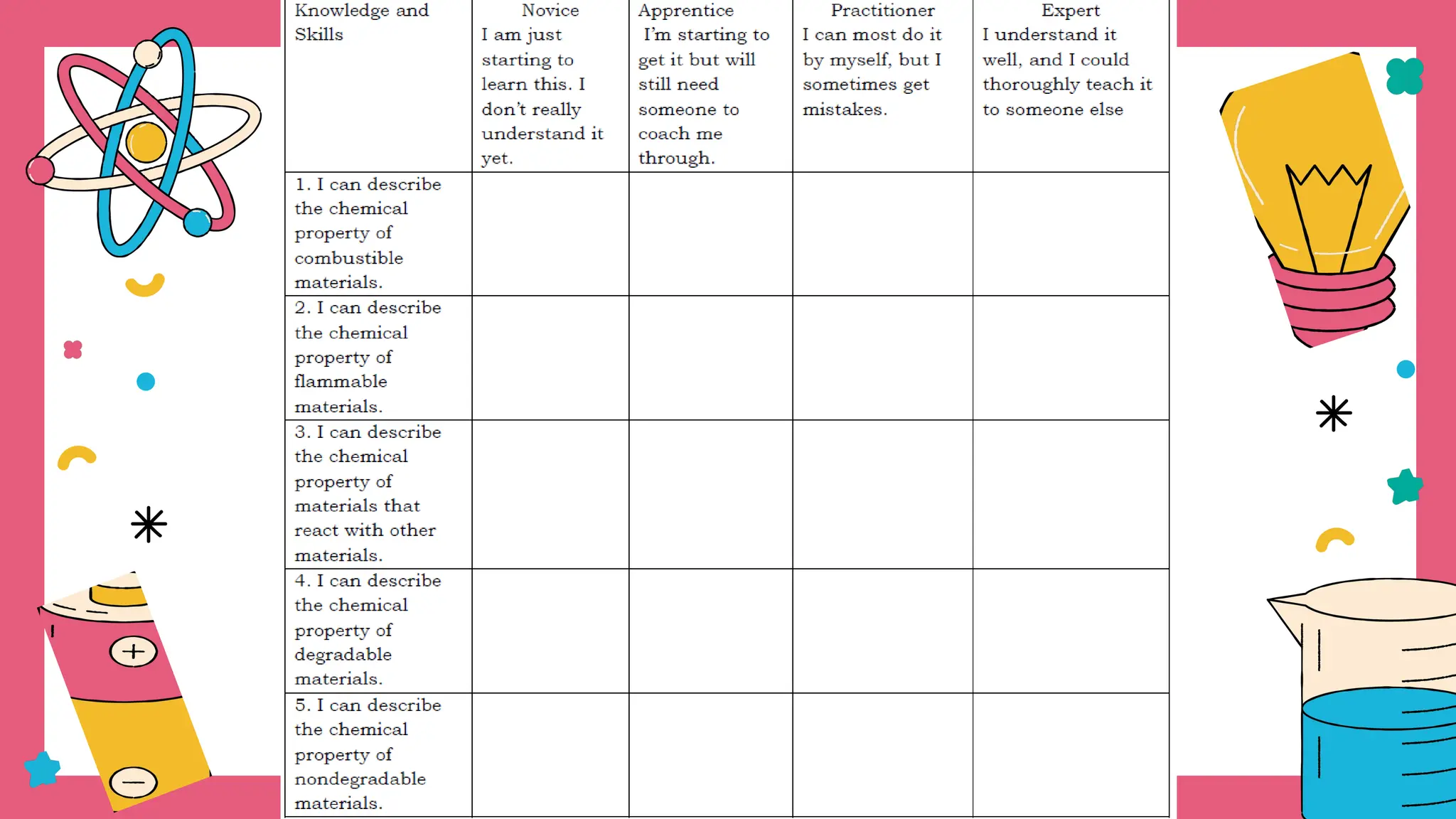
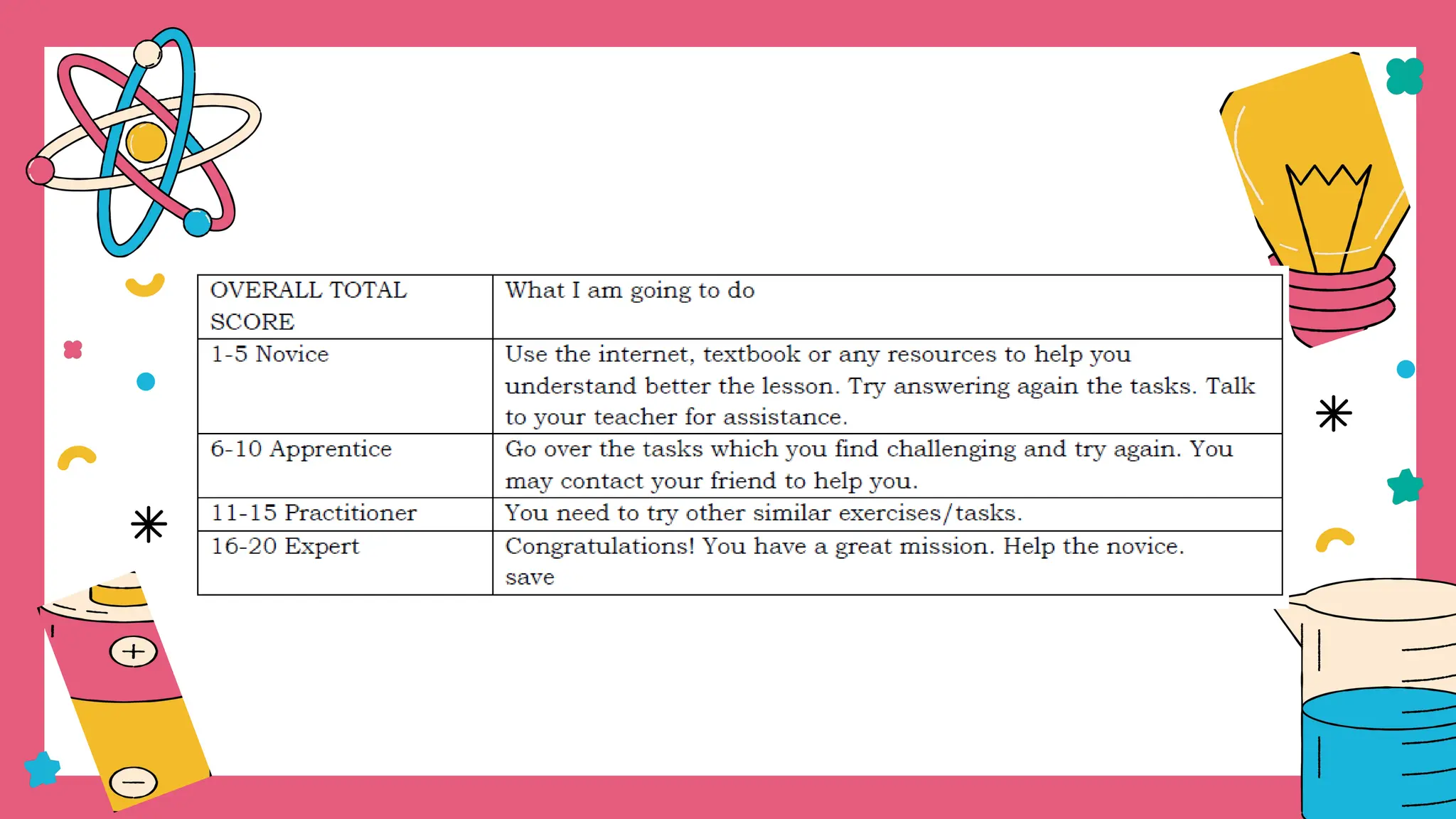
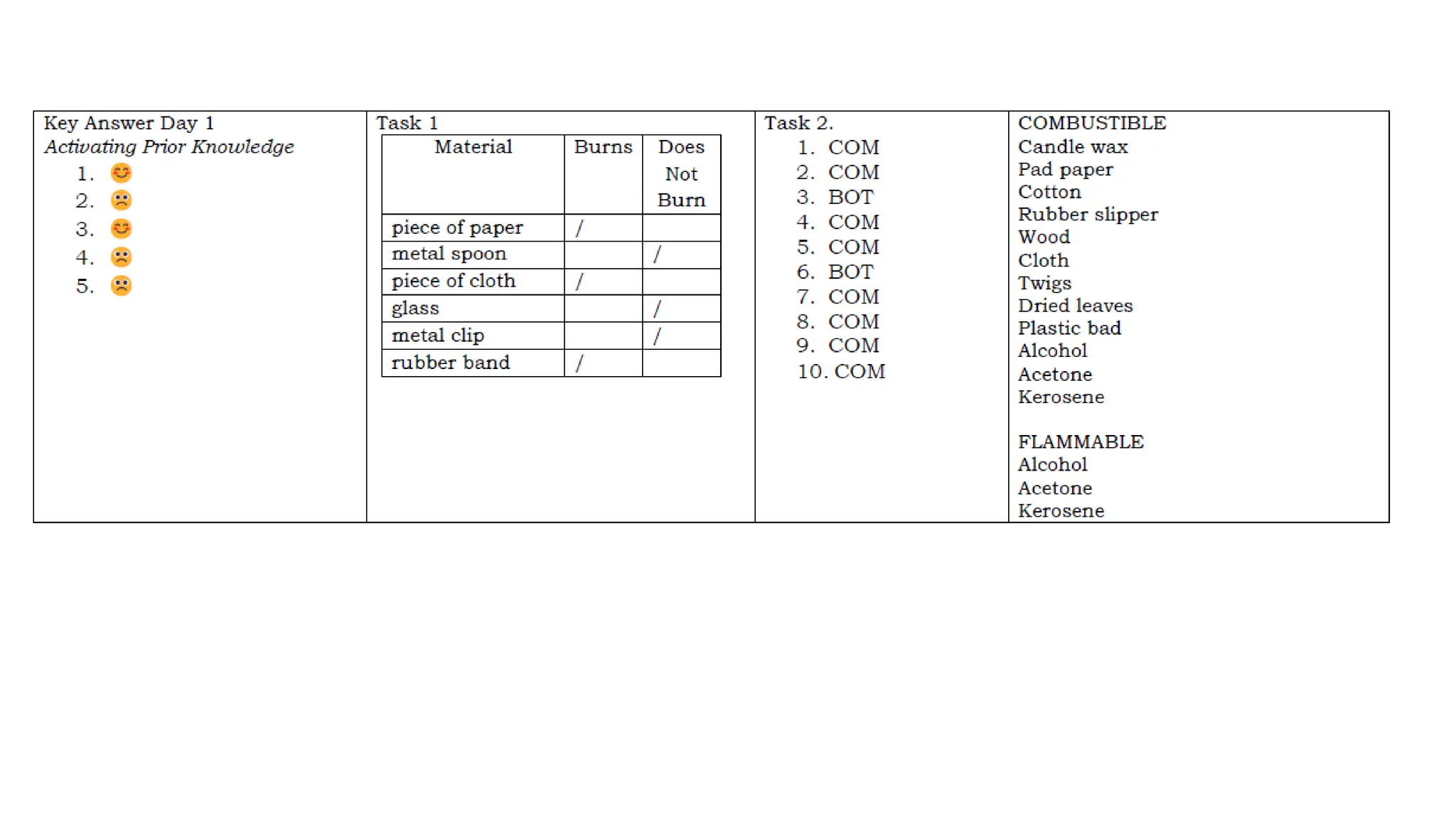
The document outlines a science curriculum focused on the chemical properties of materials, including their combustibility, flammability, degradability, and biodegradability. It provides structured lessons and activities to help students classify materials based on their chemical properties and understand their reactions with other materials. Key learning objectives include describing chemical changes, identifying materials that decay easily, and emphasizing the safety and environmental implications of handling combustible and biodegradable materials.