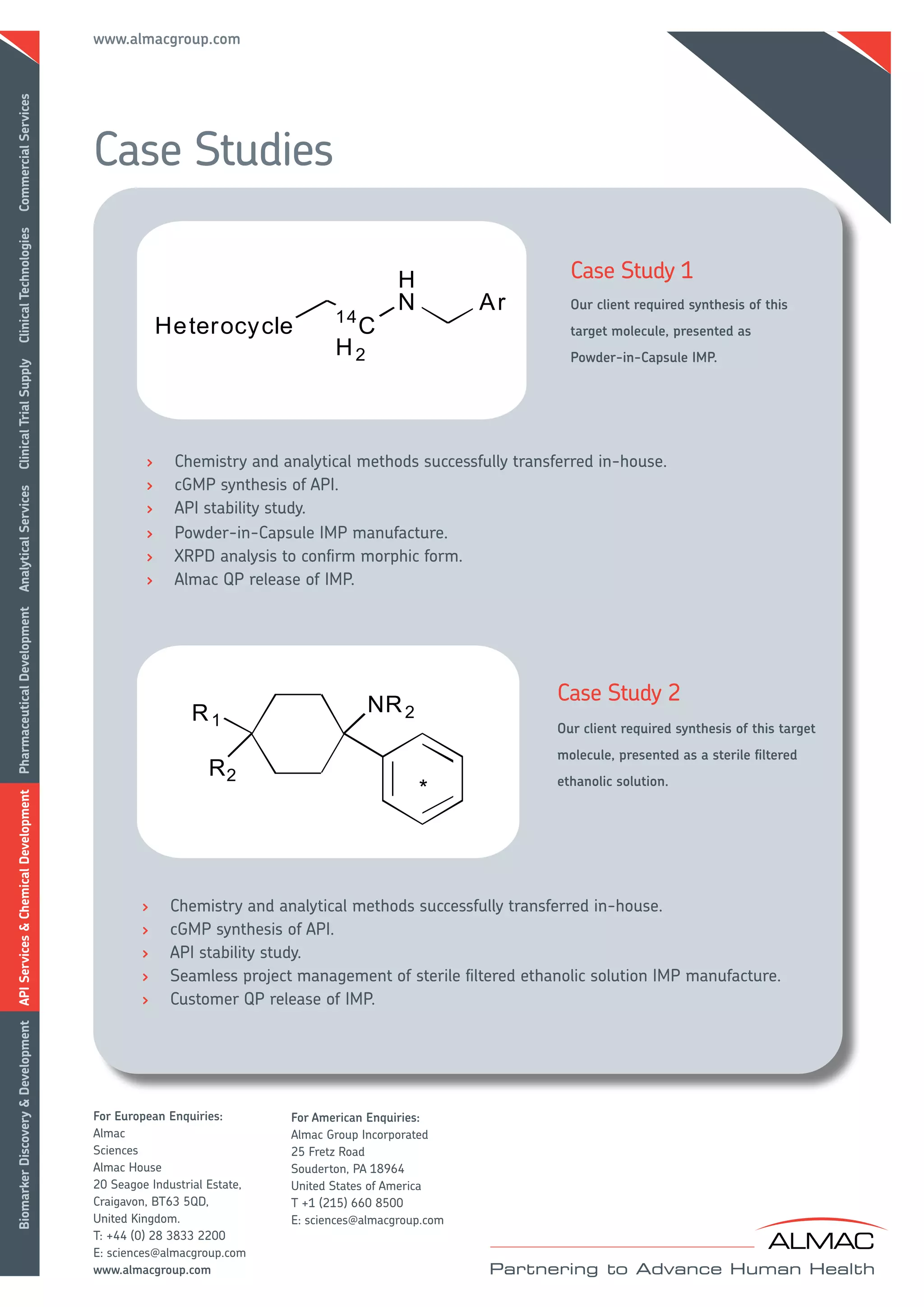
Almac provides radiolabeling services including the synthesis of 14C labeled APIs and drug products for human ADME studies. They have a 14C manufacturing license and cGMP compliance. Almac uses integrated teams of radiochemists and analytical experts along with state-of-the-art facilities to efficiently develop and manufacture radiolabeled compounds. Case studies demonstrate successful projects involving peptide radiolabeling and developing powder-filled capsules and sterile solutions for clinical studies.