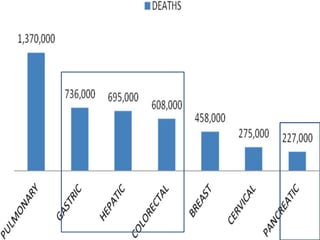
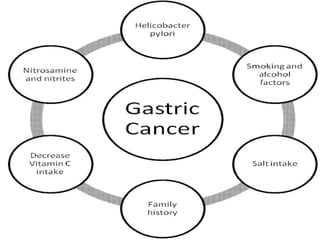
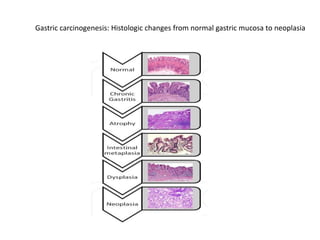
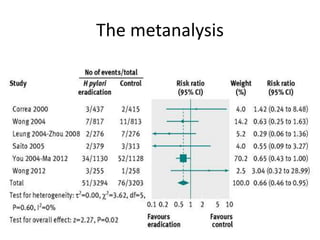
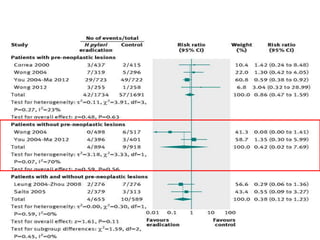
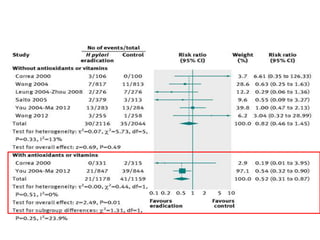
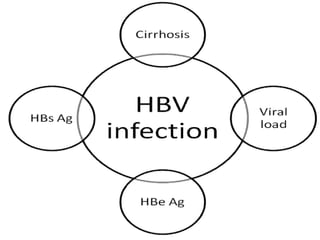
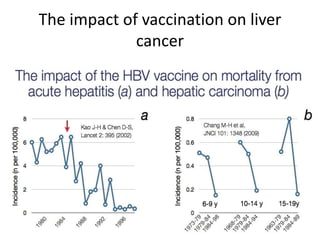
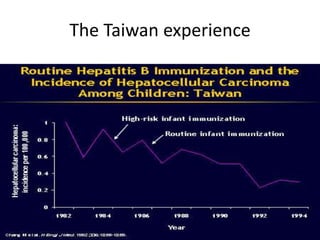
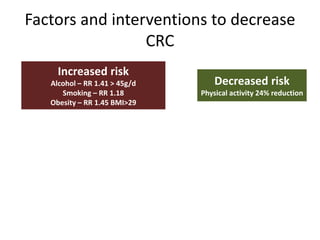
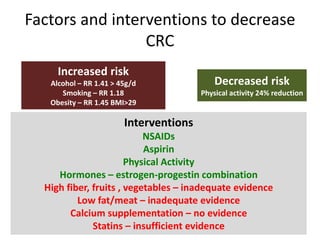
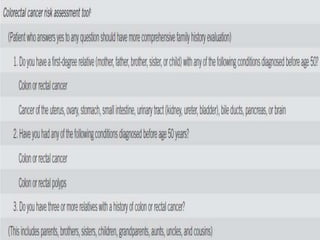
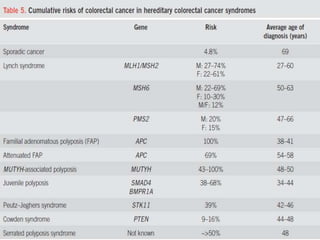
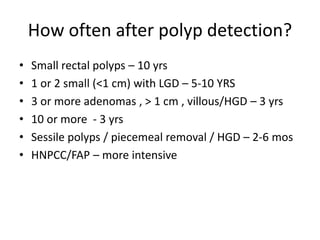
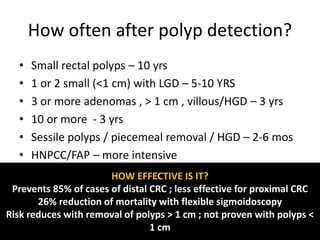
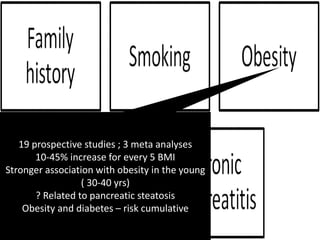
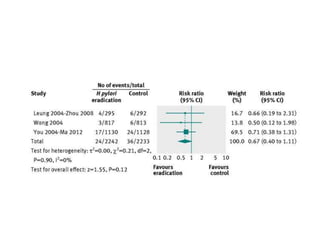
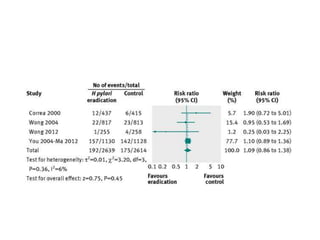
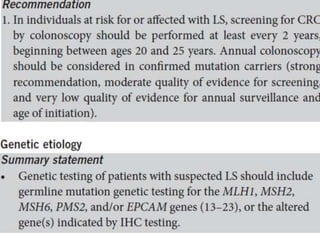
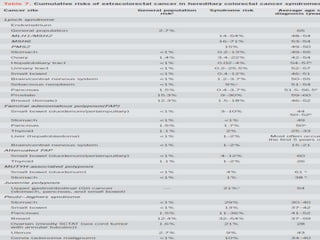
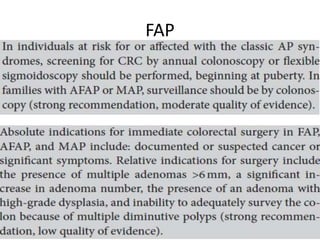
This document discusses strategies for preventing gastrointestinal (GI) cancers. It outlines several key risk factors for GI cancers, including H. pylori infection for gastric cancer, obesity for pancreatic cancer, and family history for colon and gastric cancers. Prevention strategies discussed include H. pylori eradication, vaccination for hepatitis B, lifestyle modifications like diet and exercise, and cancer screening programs. The document emphasizes that while knowledge of risk factors is sound, interventions need improved implementation and awareness and education are critical to reducing the burden of GI cancers.