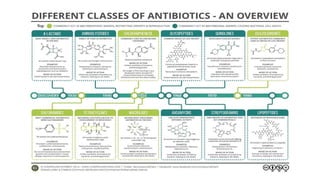
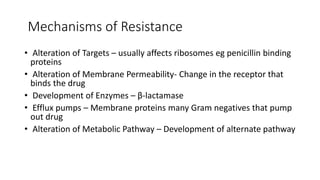
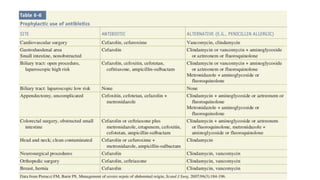
The document discusses the judicious use of antibiotics, including their history, definitions, classes, mechanisms of action, and factors leading to resistance. It emphasizes the importance of appropriate antibiotic use to minimize emerging infections and resistant pathogens. Key recommendations include using narrow-spectrum antibiotics based on diagnosis and culture results, adhering to proper dosage and duration, and implementing antibiotic stewardship and infection control programs. Antibiotic prophylaxis and treatment are outlined for different surgical case classifications and indications.