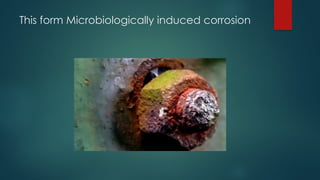
The document discusses various types of corrosion, including galvanic corrosion, stress corrosion cracking (SCC), and microbiologically induced corrosion (MIC). Each type is characterized by its causes and effects on metal structures, particularly in environmental contexts like oil platforms. Strategies for preventing these forms of corrosion are also highlighted, such as using insulation, controlling stress levels, and employing chemical treatments.