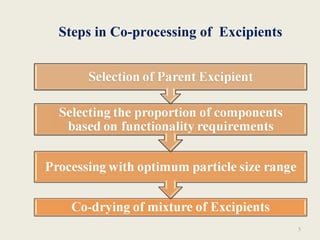
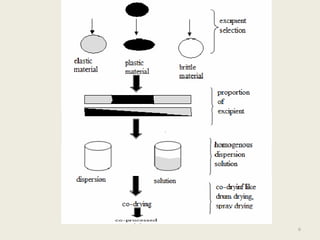
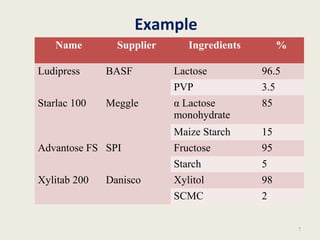
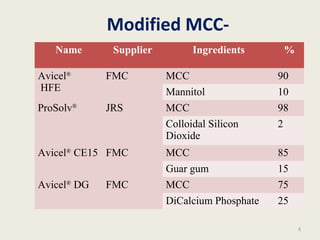
This document summarizes a seminar presentation on co-processed excipients. It defines co-processing as interacting two or more excipients at the sub-particle level to provide functional synergies and mask undesirable properties. The goals of co-processing are to obtain an excipient with added value and cost-effectiveness. Co-processed excipients can improve flow, compressibility, solubility, and disintegration properties. Examples of co-processed excipients are provided along with their advantages such as multiple functionalities, better palatability, and removal of undesirable properties.