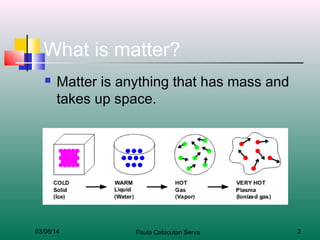
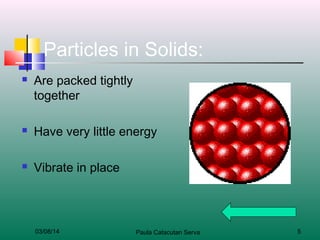
The document discusses the four states of matter: solids, liquids, gases, and plasma. It describes the key properties of each state, including how the particles are arranged and their energy levels. Solids have a definite shape and volume, and particles vibrate in place. Liquids take the shape of their container but have definite volume, and particles flow around each other. Gases spread out to fill their container without a definite volume, and particles move freely. Plasma is similar to gas but with electrically charged particles. The level of particle energy determines the state, and changing energy levels can cause a state change through processes like melting or boiling.