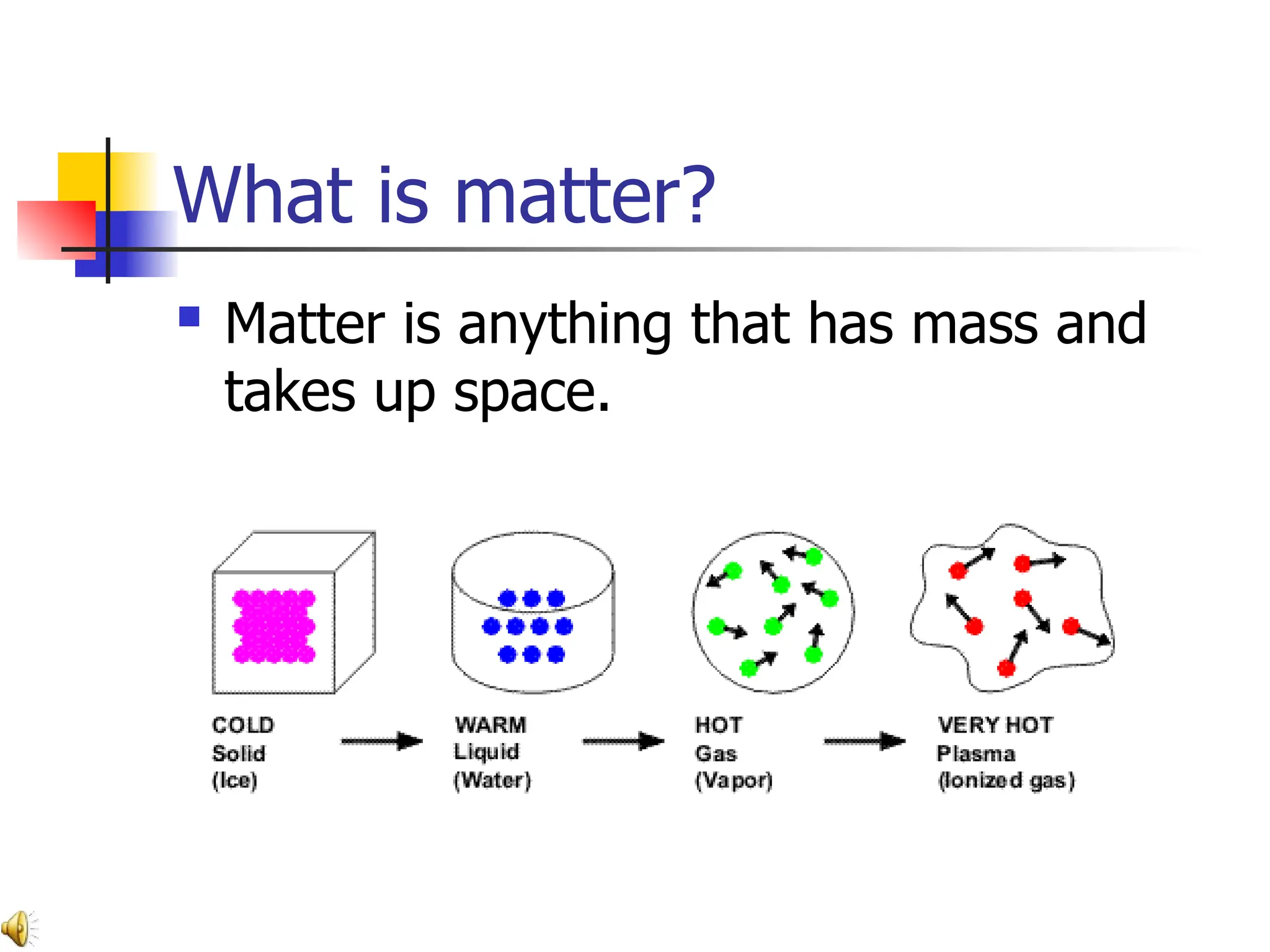
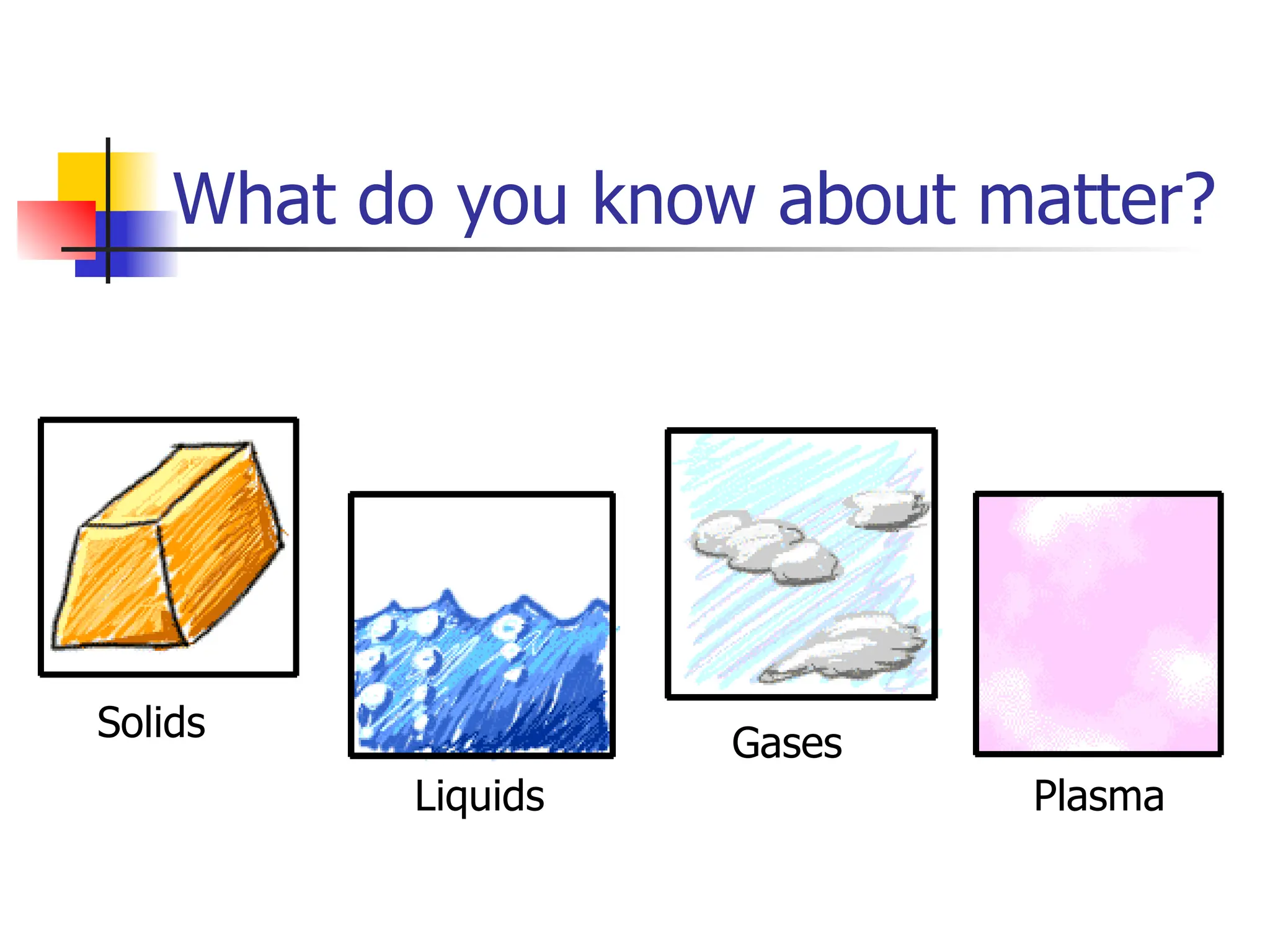
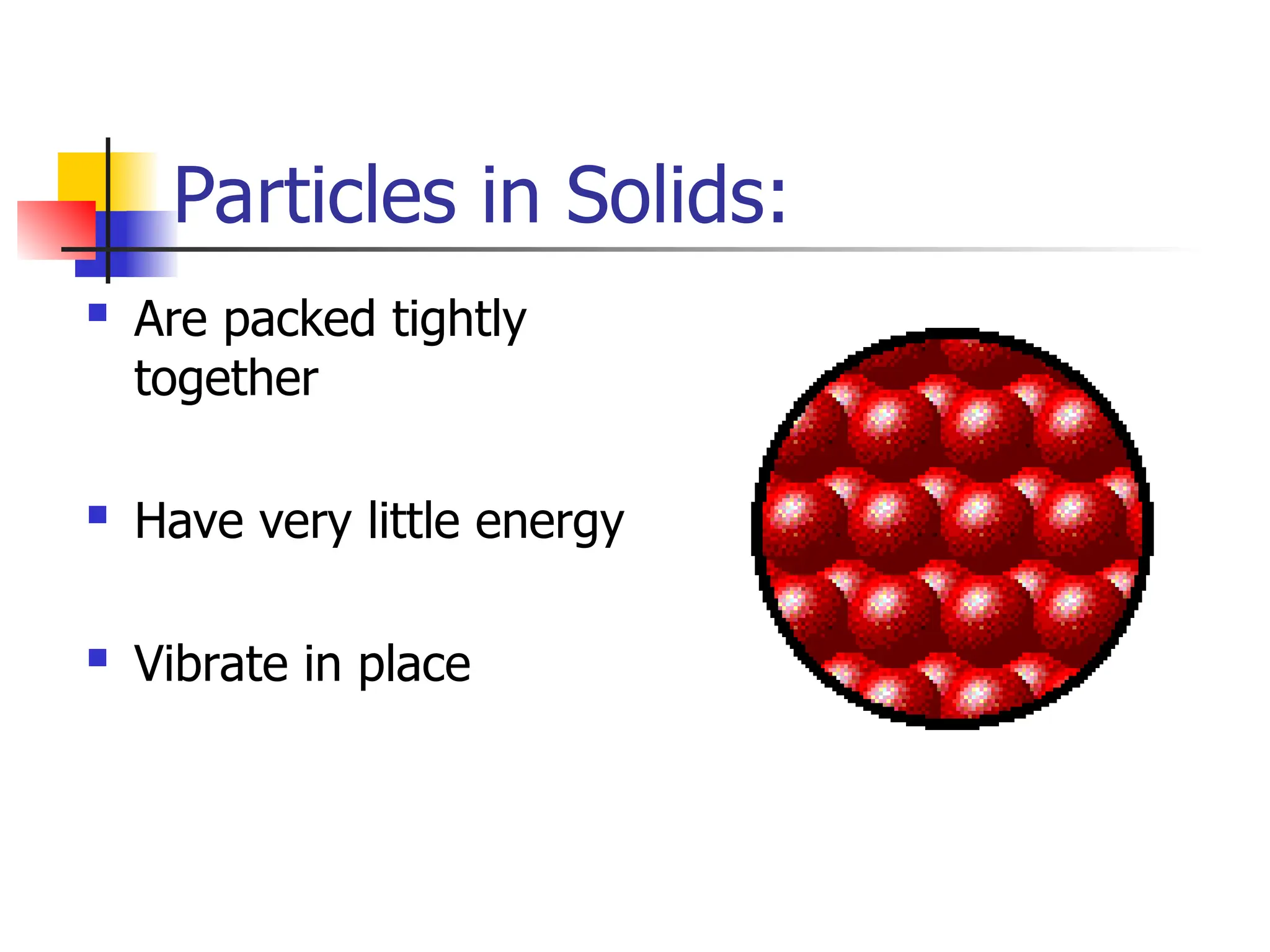
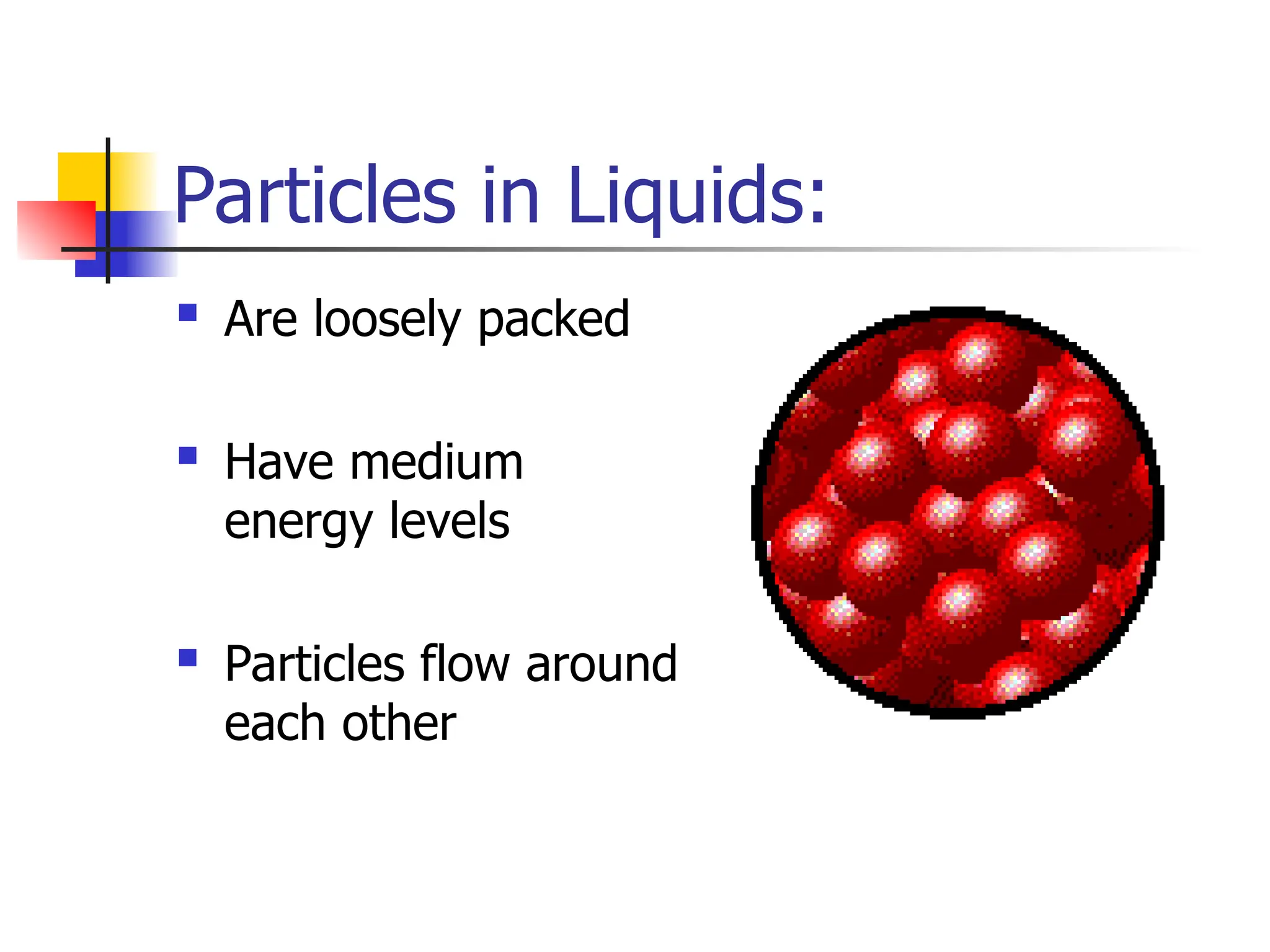
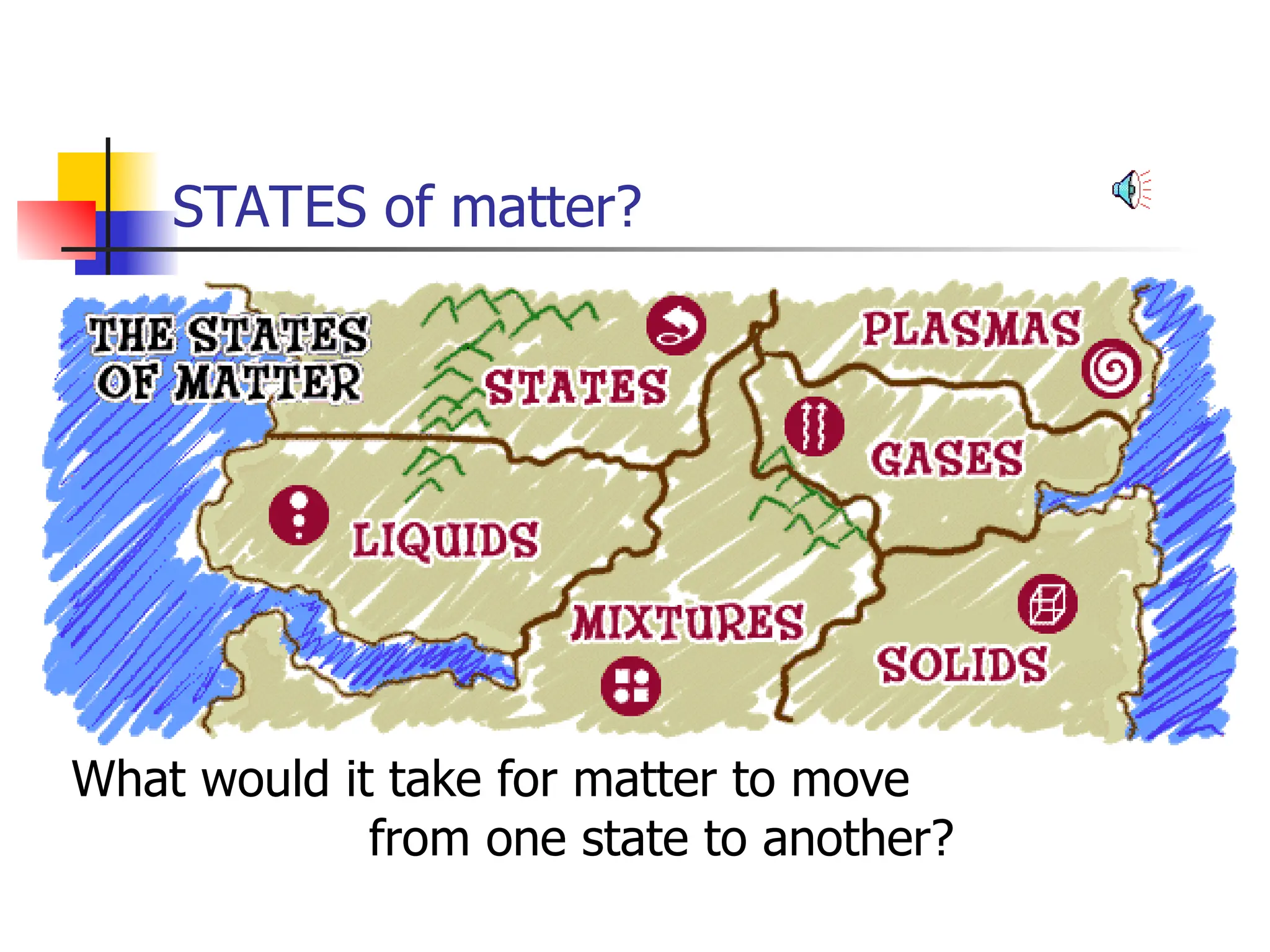
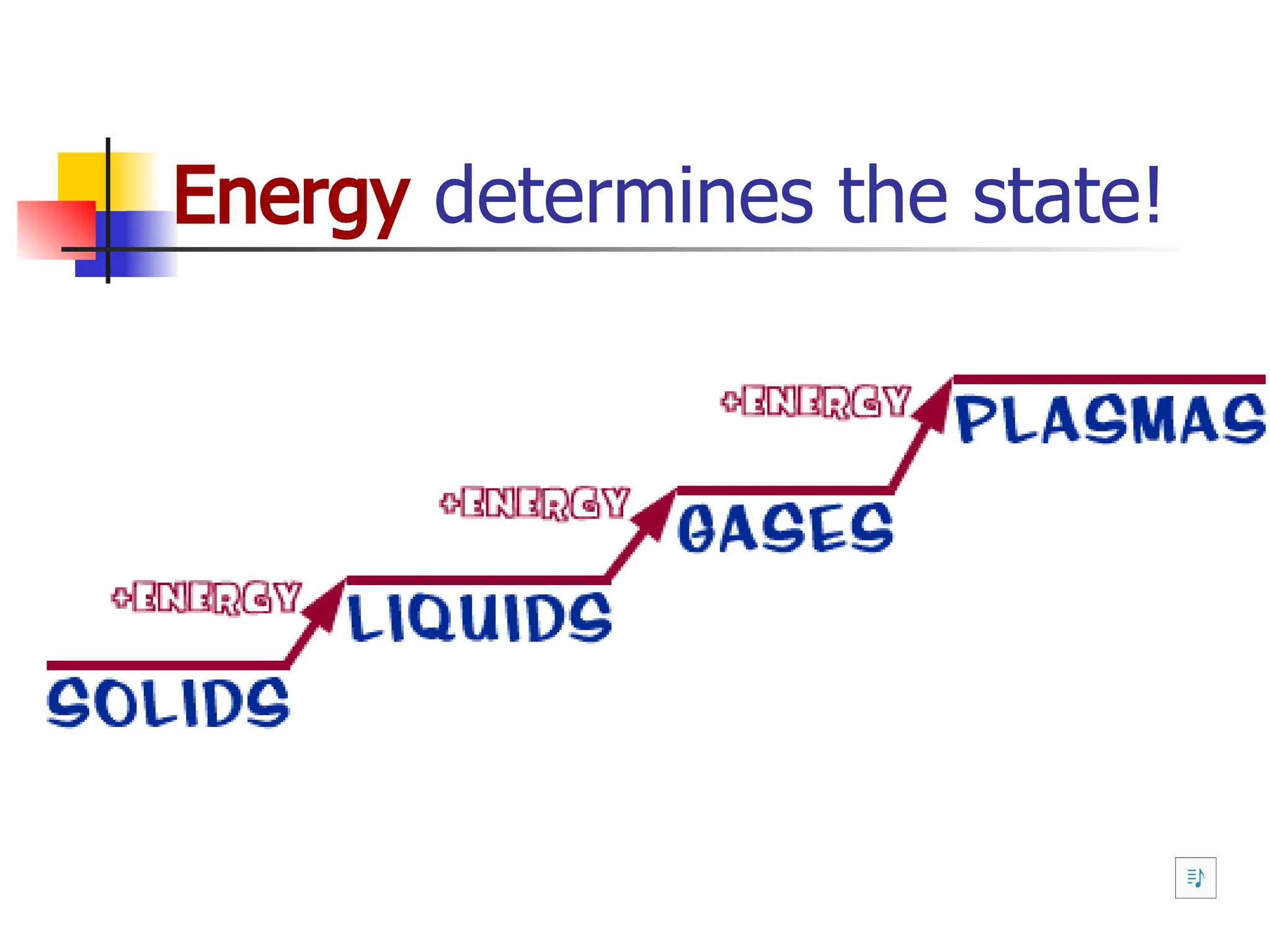
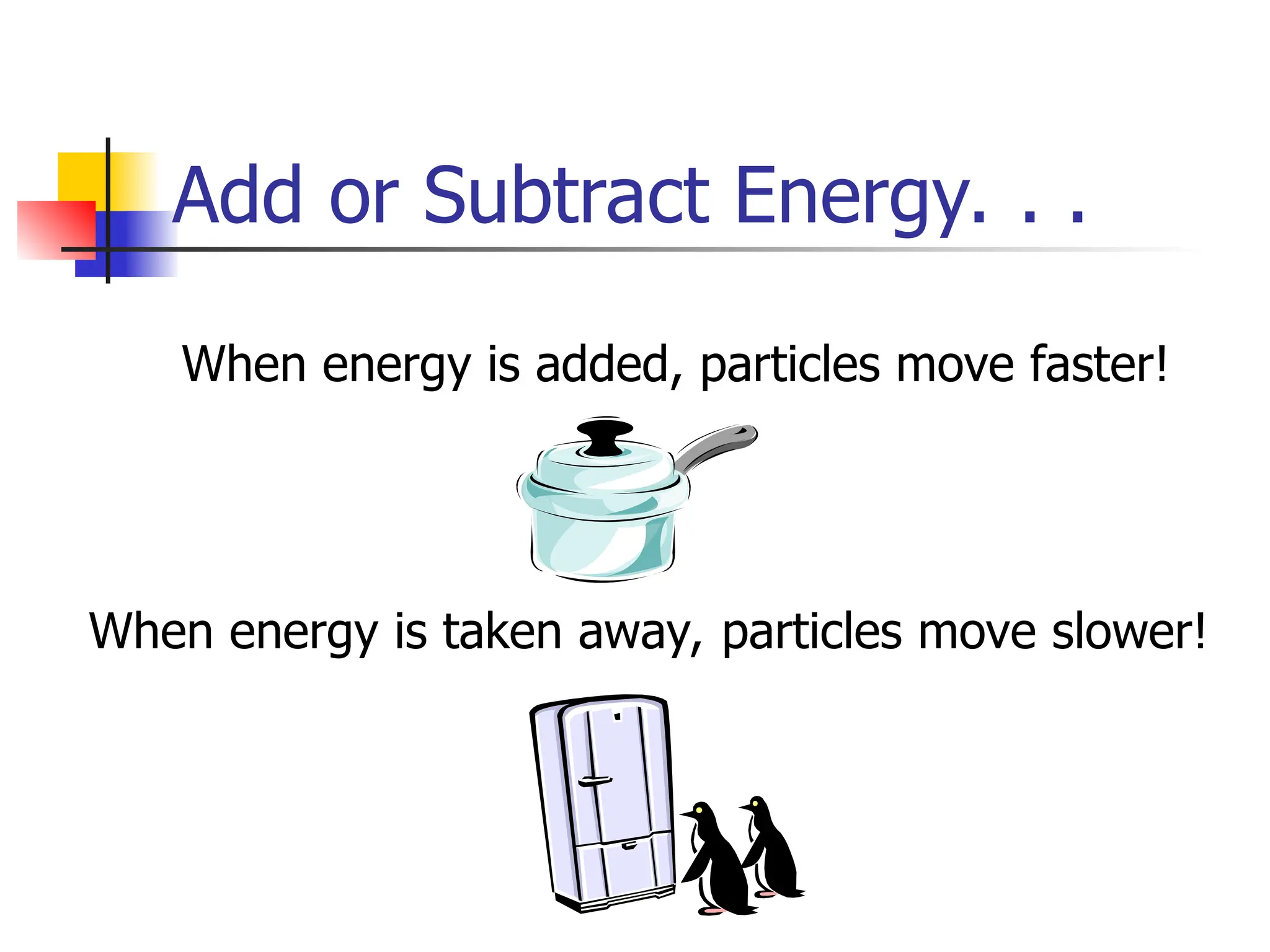
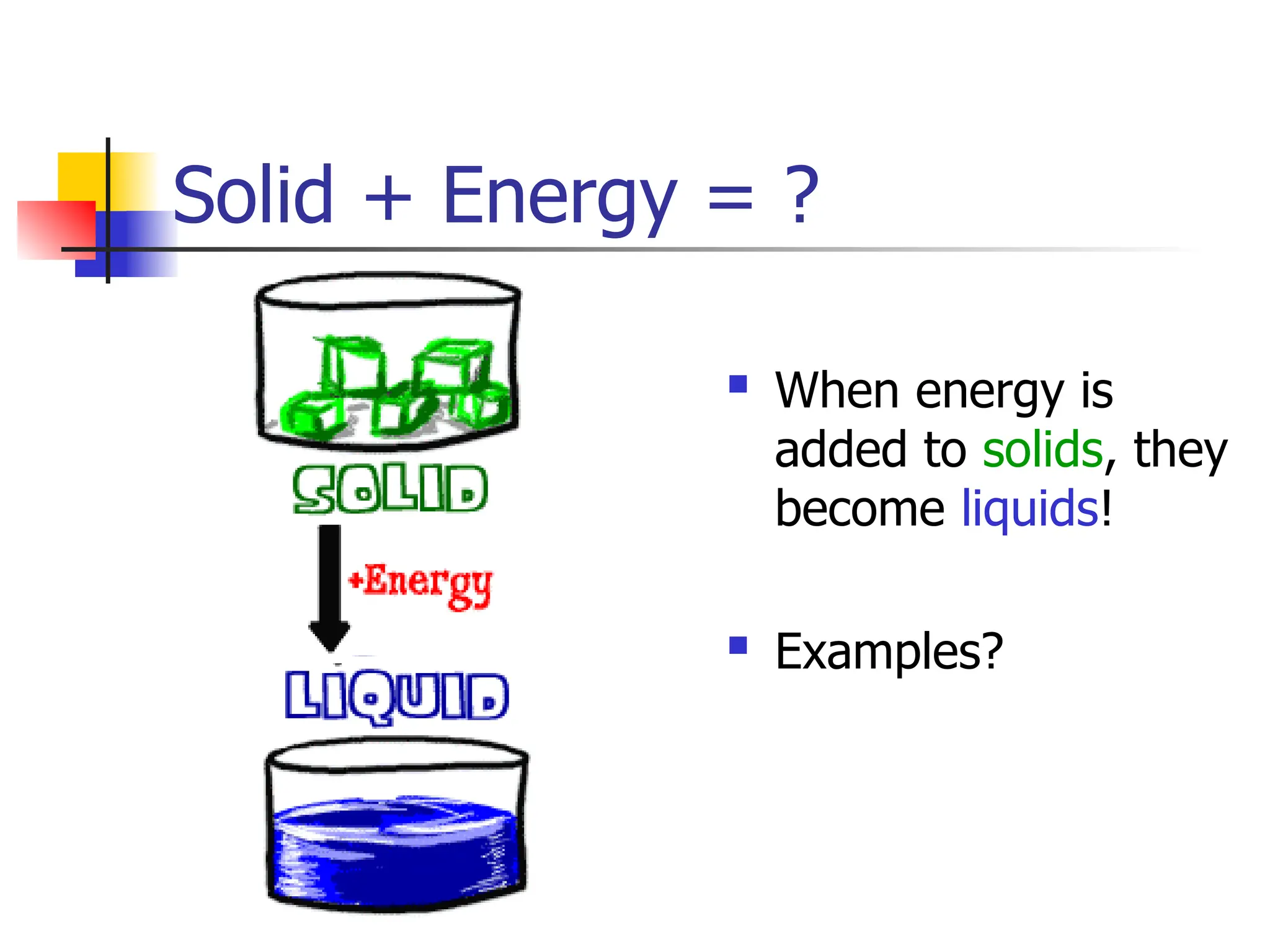
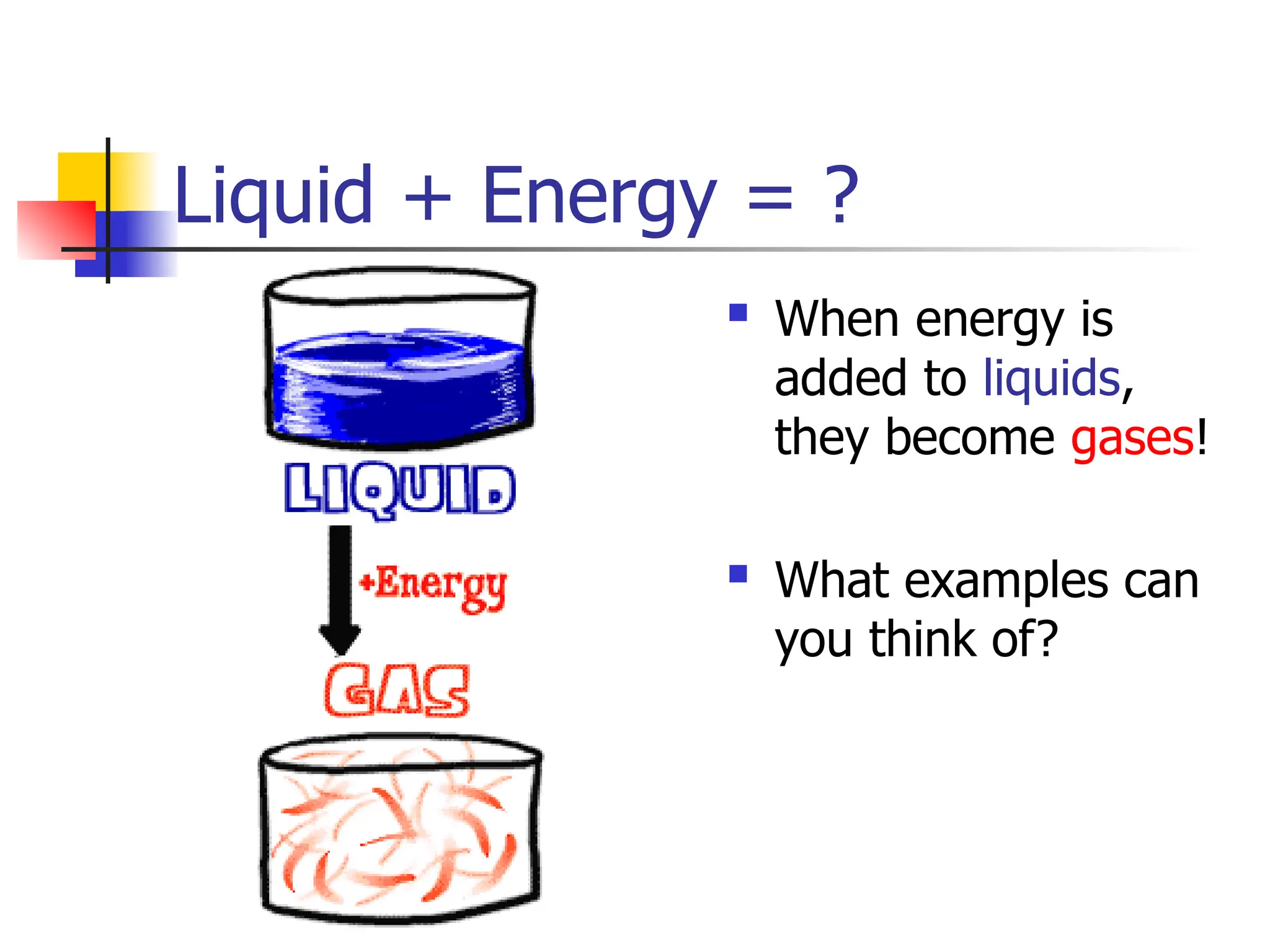
Matter is anything that has mass and occupies space, and it exists in four states: solids, liquids, gases, and plasma, each with distinct properties. The states differ in terms of particle arrangement and energy levels, with solids being tightly packed, liquids flowing, gases spreading out, and plasma consisting of charged particles with high energy. The state of matter can change through the addition or removal of energy, leading to phase changes such as solids turning into liquids or gases.