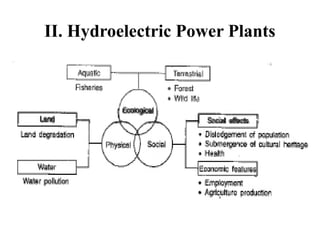
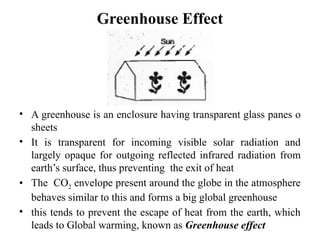
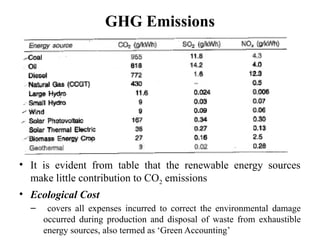
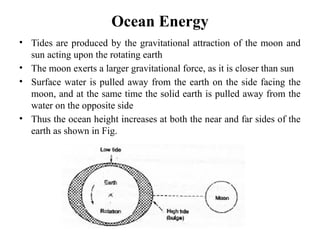
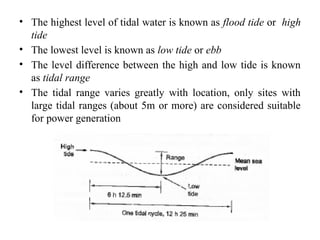
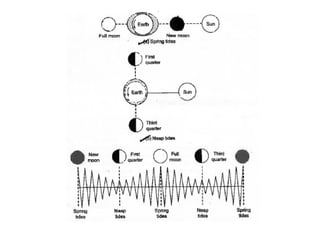
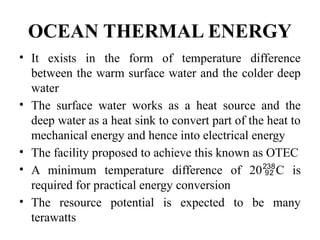
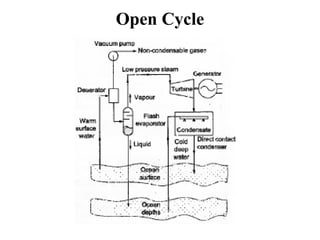
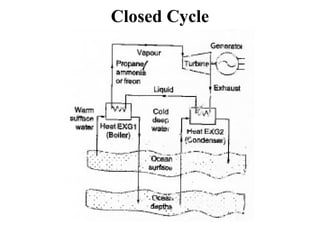
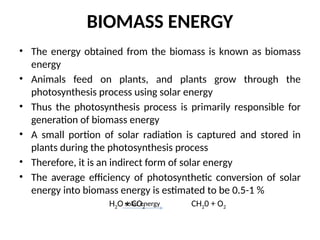
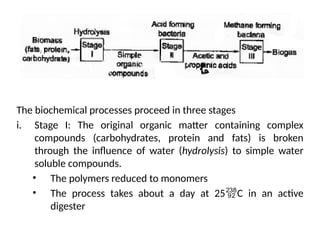
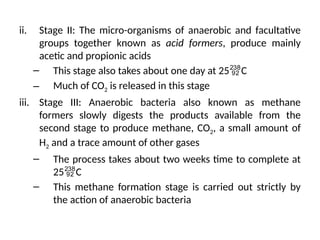
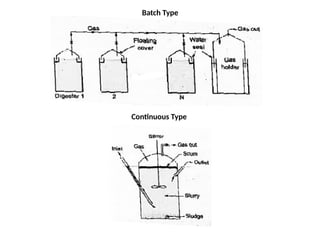
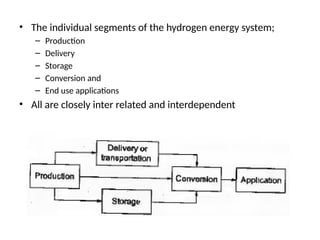
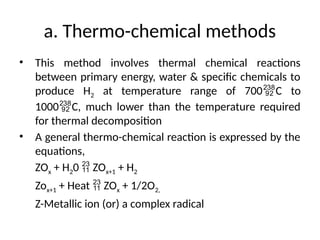
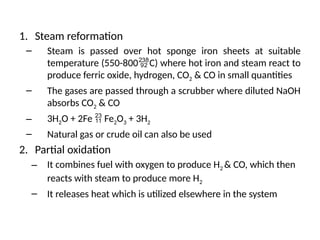
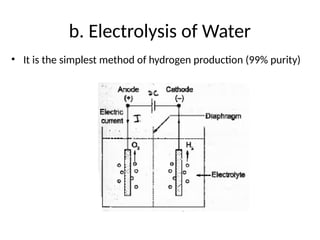
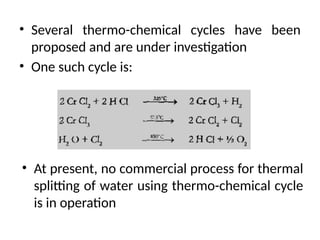
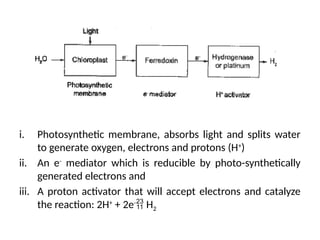
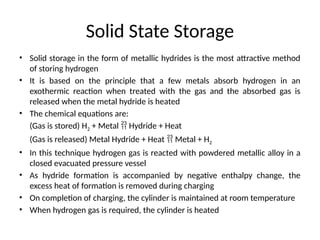
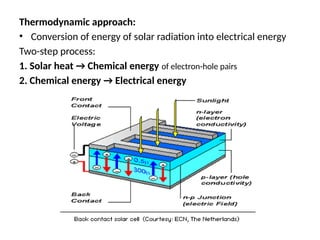
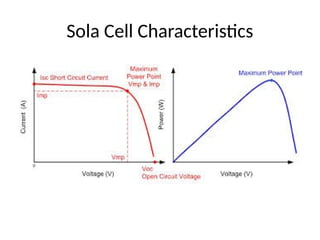
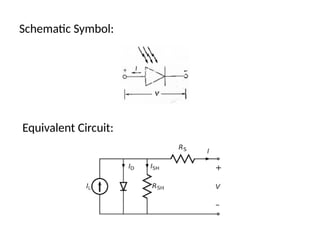
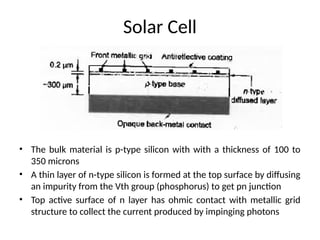
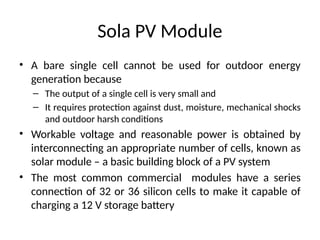
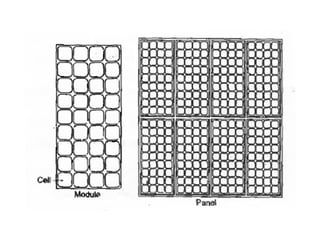
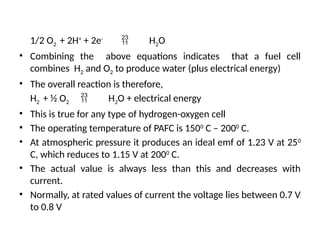
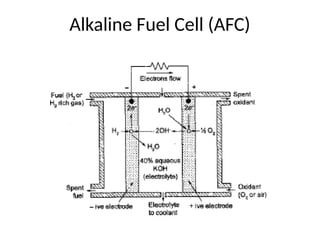
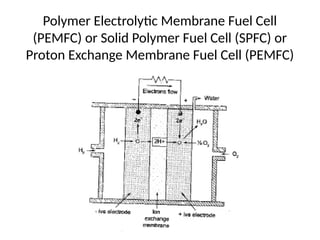
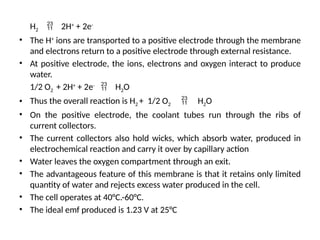
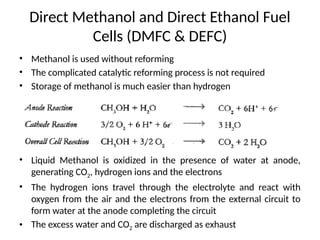
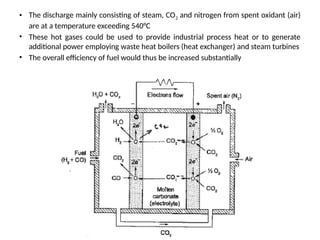
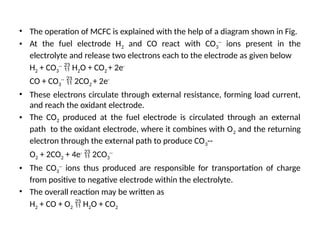
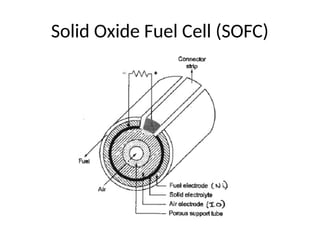
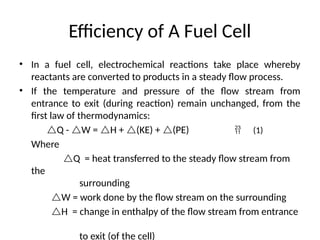
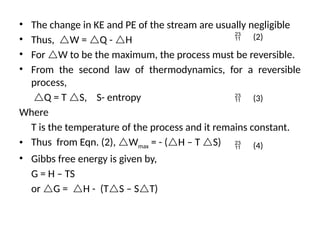
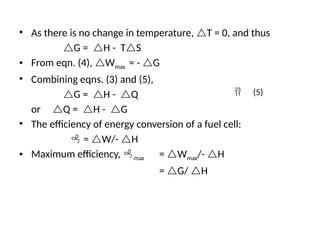
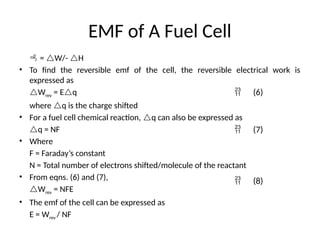
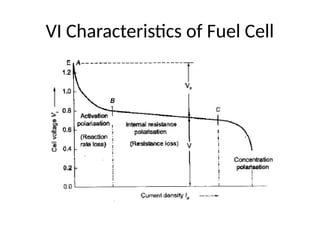
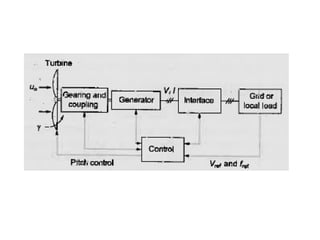
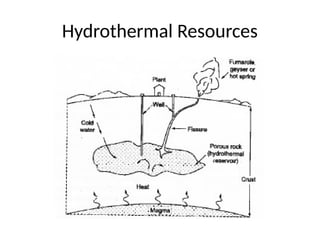
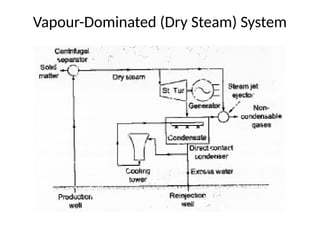
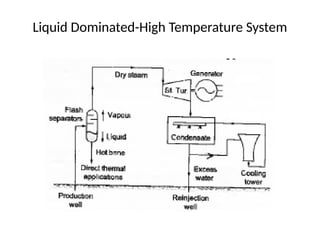
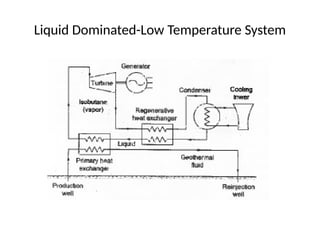
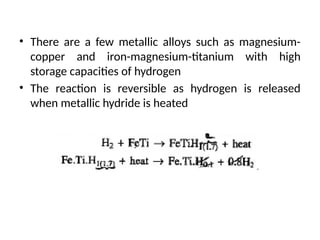The document outlines a syllabus for a course on power electronics for renewable energy systems, covering various units from introduction to hybrid systems. It addresses the environmental impacts of electricity generation and renewable resources, detailing harmful emissions and ecological consequences associated with thermal, hydroelectric, nuclear, and renewable energy sources. The document also discusses energy cost comparisons, global warming effects, and innovative technologies like ocean thermal energy conversion (OTEC), emphasizing the need for sustainable practices in energy production.