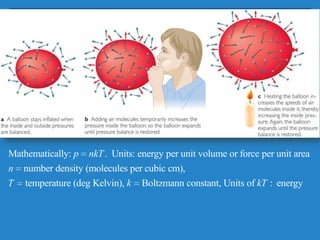
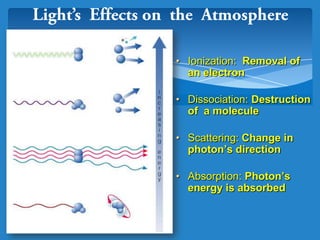
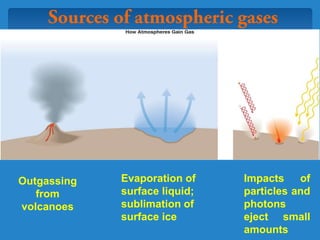
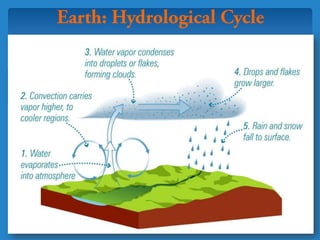
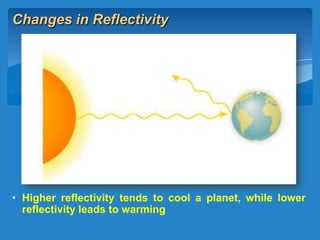
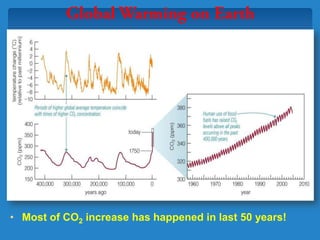
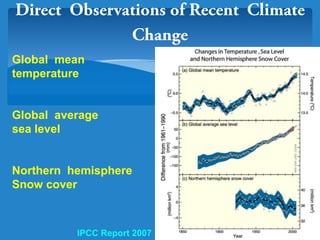
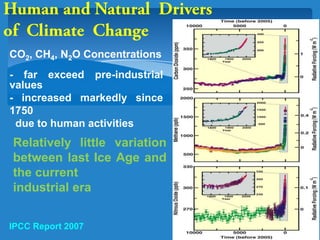
The document discusses the atmospheres of terrestrial planets. It begins by defining what an atmosphere is and its basic structure. It then discusses atmospheric structure and composition for Earth, Venus, and Mars. Key points are made about how planetary atmospheres developed over time based on interactions between gravity, heating from the sun, and geological processes like volcanism. The document notes that atmospheric conditions on these planets have changed dramatically since their formations.